Category: "Snow Science"
Bentley’s Most Singular Observation
March 2nd, 2022[This is the seventh and last in the series of re-posted articles, from 2012.]
You don’t have to look at frosted surfaces for very long before coming across something like the following.
The picture shows a large ice crystal amid a roughly uniform sea of tiny frozen droplets. Between the large crystal and the frozen droplets lies a clear ice-free zone, a dry moat around an island of ice. Sometime prior to 1907, the Vermont farmer Wilson A. Bentley took notice of this moat. Writing in the Monthly Weather Review in 1907, he wrote
"One of the most singular, and doubtless most important, phenomena that occur in connection with the formation of window frost is this: The true crystalline varieties of window frost ordinarily, apparently, repel the minute liquid particles or droplets of water that frequently collect like tiny dew-drops on the glass, and freeze in granular form thereon."
Why six?
March 2nd, 2022[This is the sixth in the series of re-posted articles, from 2011.]
Why do so many snow crystals look about the same when rotated by 1/6 of a turn? What’s the origin of the six-fold symmetry? Why not five or seven, as Kepler asked(1)?
Since it always happens, when it begins to snow, that the first particles of snow adopt the shape of small, six-cornered stars, there must be a particular cause; for if it happened by chance, why would they always fall with six corners, and not with five, or seven, as long as they are still scattered and distinct, and before they are driven into a confused mass?
Though we can now answer the last two questions, the first, as pondered by Bentley and many others, still awaits a complete answer.
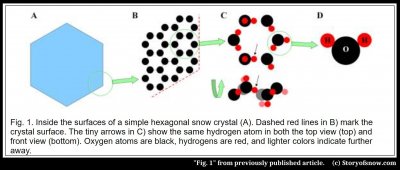
The thing that allows (but does NOT cause) the crystal to have six-fold symmetry comes down to in its internal crystalline lattice of water molecules. Specifically, if you could zoom in about a million times into any region of a snow crystal, such as the corner in figure 1A below, you would see a lattice of hexagonal rings B) – like a microscopic internal honeycomb. The oxygen atoms (black) in each ring have the six-fold symmetry. But if you further examine the rings, notice that all of them are rotated by 30º in relation to the crystal hexagon in A).
However, if you look even closer, such that you can see the orientations of the molecules, and turn the ring on its side, as in C), you will see that in fact the ring is not perfectly six-fold symmetric and it’s not even flat! The honeycomb inside ice is not so simple. But if you sought the overly simplified answer to the origin of the “6”, find it in the hexagonal lattice. Call that the answer for the easily persuaded. There are serious problems though with that answer.
The simple answer specifically has two problems. The internal honeycomb just gives us a similarity between the ice lattice and the crystal form – it suggests that the crystal may develop six-fold symmetry. But just because a crystal may have six sides doesn’t mean it will have six sides. Or, to use one of Kepler’s examples, a beehive also has an internal honeycomb structure, and yet from the outside it appears blobby and nondescript – hardly six-sided.
Blues and Whites of Snow and Ice
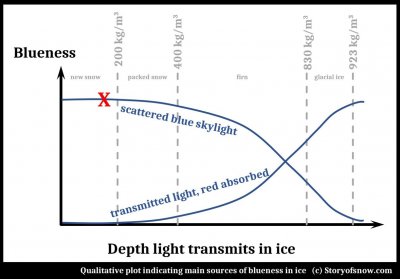
March 1st, 2022A recent article in the local newspaper asks the question "Why does snow glow blue?"
The author gave one inspiration for the article as "..the way white snow glows turquoise in the holes left by boots or ski poles..."
The explanation given in the article is the same as that you can find elsewhere on the internet, and certainly applies to glacial ice: absorption of the red end of the solar spectrum upon passing through ice. Does it indeed apply to "holes left by boots or ski poles" in snow?
(The answer is "Not in fresh, light snow, though possible in more compressed or wetter snow." And for some quirk of this blog software, I cannot put the following plot in its proper location further down, so I place it here. Please ignore it for now and refer to it at the end of this post.)
Snow Crystal Variety and the Bentley Length Scale in Clouds
February 22nd, 2022[This is the fourth of the re-posted articles, from 2009.]
“… by means of these wonderfully delicate and exquisite figures, much may be learned of the history of each crystal and the changes through which it has passed in its journey through cloudland.”
W. A. Bentley, 1898
“… the crystals will in all probability be greatly modified by passing through atmospheric strata varying so greatly in density, temperature, humidity, etc. That they are greatly modified by these flights in the cloud is clearly shown by the interior structure of many of the crystals. ”
W. A. Bentley, 1901
Long ago, Wilson Bentley argued that the various layers (‘strata’) of air that a snow crystal falls through largely determine the appearance of the crystal. The first direct support of this claim came about 30 years later, from laboratory experiments by Ukichiro Nakaya, a Japanese physicist. Now, 100 years later, probably all cloud scientists would agree with Bentley. So, with all the crystal and cloud research that has been done since Bentley and Nakaya, can we determine the history of a crystal if we see its form? Can we finally do what Bentley said we should be able to do?
The Snowflake’s Closest of Kin
February 21st, 2022[From 2006 through 2012, I contributed annual articles to the annual newsletter "Snow Crystals" for the Wilson Bentley Historical Society. That newsletter is no longer available, so I will repost my articles here, starting with this one from 2006.]
Wilson Bentley is well known to readers here for his photomicrographs of snow crystals. Snow, however, was only one of the many ‘water wonders’ that held his fascination (ref. 1). Some of these wonders were made of liquid water, such as dew, and some, like the snow crystal, were frozen water (ice).
The frozen type he called “The snowflake’s closest of kin”, and they included hoarfrost, rime, windowpane frost, and ice flowers (2). To obtain photographs of any of them with the quality obtained by Bentley is difficult even now, which is yet another reason to admire Bentley’s skill and perseverance.
On the inside, these ‘kin’ all have the same crystal structure. But they appear different on the outside, largely due to the different ways the water in the surroundings gets to the ice surface. There are many distinct kin because the surrounding water can be in various states (i.e., ice, liquid, and vapor) and there are many ways that each state of water can get to the ice surface. I’ll focus here on snow crystals, hoarfrost, rime, windowpane frost, and ice flowers. These forms are commonly seen by many of us, and have been observed by people for a very long time. So it is easy to think, as I probably once did, that they are well understood by science. But this view is quite mistaken. Yes, we know they all consist of H2O molecules and we know something about the structure of ice, but how exactly they form contain many mysteries. I’ll describe briefly what Bentley thought of them, and what I think is known and not known about them.
Capturing Falling Snow in a Cold Fluid
February 15th, 2021Snow is usually imaged in air, the single crystals laying flat on some substrate such as glass. The method is relatively simple, but one must work fast to image the crystal before it appreciably sublimates. Sublimation first rounds out the sharp edges and then causes the crystal to shrink. Generally, this sublimation happens because the photographer is radiating too much heat to the crystal. Conversely, particularly in very cold conditions, the photographer’s breath may deposit fog near the crystal, causing the crystal to grow.
Such issues vanish if one instead captures the snow in a cold fluid before taking the image. To work, this fluid should not dissolve the ice, be less dense than ice, be fluid enough to completely spread over the crystal surface, and be transparent. Other than preserving the crystal, the method has several other advantages. For example, in his laboratory experiments in Hokkaido, Japan, Tsuneya Takahashi lets the crystal fall into a cold suspension of two transparent, cold silicone oils. He sets it up so one fluid is denser than ice, one is lighter than ice, so the crystal falls through the light oil and rests on the (transparent) interface between the two fluids.
This method sounds complicated, so why use it? One, as the oils are immiscible with water, they block water molecules from arriving or leaving the crystal surfaces, so the ice crystal shape is preserved precisely for as long as the fluid is below 32 F (0 C). Two, after imaging the crystal, the fluid is warmed above melting such that the crystal melts into a spherical drop from which he can easily measure the volume and thus infer the mass of the original crystal. A third advantage, more difficult to exploit yet sometimes used, is that he can get top and side views of the same crystal. Other researchers in Japan have also used silicone oils to capture ice crystals in the lab, as well as naturally falling crystals, mainly for the first and third reason. They use just one oil type, a lighter oil. Charles Knight at NCAR in Boulder, Colorado had a fourth reason for using a cold fluid: better imaging. That is, one can image greater depth detail because light scattering off the surfaces is greatly reduced, particularly if the fluid is very clear and has an index of refraction close to that of ice. By reducing the scattering, one can see through surfaces to underlying surfaces. He would use gasoline or hexane fluid.
I don’t have a photomicrography setup to take detailed images of snow, and we rarely get snowfall with nice single crystals anyway, but I wondered how well the method might capture falling snowflakes. That is, could I at least see their rough shapes as they fell through the fluid?
Here, we typically get about one light snowfall per winter (2-3"). A relatively large snowfall happened this past weekend, depositing about seven inches. At first, the particles were small, probably highly rimed single crystals or small aggregates. Later, larger snow particles fell, and these particles were clearly snowflakes (i.e., aggregates). In preparation, the previous night I set out two covered wide-mount jars, one with water, the other with Coleman camping fuel (white gas), which has similar properties to gasoline. In the morning, the one with water had frozen, so I knew the other was also below 32 F.
Outdoors, I set the jar on top of my car, put a wooden chopstick in the jar both to focus on (my camera only has autofocus) and to provide a size reference, set a small LED light panel to the side for brighter illumination, and then opened the lid. The flakes fell into the fluid, and fell down to the bottom of the jar. They fell through the fluid slower than they fell through the air, but it was still too fast for me to see how well they were focused. Turned out that they were not very sharply focused, yet one can still see their general shape and fall orientation (below). In general, the flatter the flake, the more it tends to orient broadside to the fall direction.
Obvious improvements would be a better jar, such as one with a flat, smooth glass front, a better camera, and a thicker fluid to slow down the rate of fall.
Such improvements will have to wait at least until next winter.
--JN
The Curious World of Ice and Snow: Part 1 of 3
February 4th, 2020In 2012, I gave a "science cafe" talk with a local series sponsored by the Pacific Science Center, KCTS public television, and Science on Tap. The title was "The curious world of ice and snow". The location was a bar in Kirkland, but open to all ages. When I showed up with my family, they tried to seat us in the backup room, the regular room having filled up, but I said "Oh, well I'm the speaker" and they kindly created a space for my family in the regular room. I was indeed surprised at the crowd. People are apparently more interested in ice than I thought. (Hmm, but where are they when I post here?)
Click on any image to see an enlargement.
The basic structure of each talk was to give a lecture of about 30 minutes and then allow up to an hour (I think) for the Q&A. In my excitement, I had created 41 slides, in retrospect too many for the allotted time.
Given all the time spent preparing the slides, I hope that by posting them here that even more folks can enjoy the images and discussions. But, instead of unloading all of them on you at once, I will break the discussion into three sections. By adding the following table of contents, each section will have 14 new slides and the total will be 42, which according to Douglas Adams* is a really special number.
The contents of this section is part "1", written in green font.
Some "Inexplicable" Snow-crystal Features: Applications of Lateral Growth
January 29th, 2020Last October, I gave a talk at the University of Washington about our recent experiments and ideas about snow-crystal growth. My pitch was general and short, as few folks work in this area and I'd hate to bore them with a long lecture. So, I was delighted to see quite a few graduate students in the audience, some of them asking good questions.
Instead of giving the narrated presentation here as a video, I will give the slides (23) with brief explanations similar to what was spoken at the talk. Narration below each slide. Skip to the ones that look interesting, and click on them to enlarge.
Martini Hoar (raise a tiny glass?)
October 19th, 2019The hoar-frost crystal shoots up like a thin, solid straw, then suddenly opens up into a cup-like shape. I have seen it often enough to give it a name: "martini hoar".
The cup can be weirdly segmented and polyhedral, but it nevertheless widens suddenly. Here are a few more (Sorry for poor photos—someday, I hope, I'll get better about photography.)
Here is a larger view of the region. Note the similar hoar coming down from the top, but without a clear view of the base.
This sudden widening feature has bothered me for awhile, but I was delighted the other day to figure out a plausible reason. My delight was made even greater because the reason involved measurements I made in the lab two decades back. The measurements were to understand snow-crystal habit, but apply equally well to hoar frost because hoar grows just like snow except it is attached to the ground.
Now that I have viewed some of the older pictures I took, the actual growth phenomenon looks more complicated in terms of crystal shape, so I am not so sure my reasoning explains things so simply. Nevertheless, it should apply well to many cases, and at least is worth learning because it involves important growth principles that also apply to snow.
Thinking Laterally in Crystal Growth…and in Science Publishing
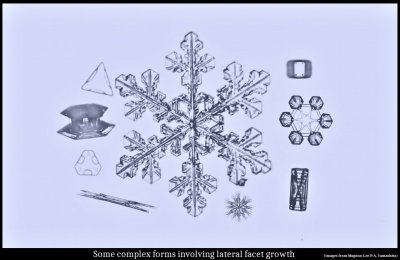
October 10th, 2019After a long period of work (on and off), we have an accepted paper on the corner pockets we discovered (see here). But during the writing stage, I thought about collecting earlier ideas I had developed during my correspondence with Prof. Akira Yamashita in Japan, and as a result, the paper ballooned. Originally, it was to be a short note about the pockets, but in the end, the central theme was instead the new notion of the lateral (or sideways) growth of crystal facets. Actually, one type of lateral growth had been long known, sometimes called "facet spreading", but we collected together three types of lateral growth and describe how two of them are particularly useful for explaining a wide range of observed features on snow and ice crystals.
But before describing some of our findings, recall that all science publishing costs money. Almost always the research grant pays the page charges. However, our grant ran out three years ago, and this paper is particularly expensive (estimate: $3600) because we needed many pages to argue our points and show how lateral growth can explain many growth forms. If you can contribute, we will gladly send a signed copy of the paper acknowledging your help. Or, if you have just enjoyed some of the articles on this blog and would like to help me continue, you can contribute here as well. Here is the link:
https://www.gofundme.com/f/publish-a-research-paper-on-snowcrystal-formation
The paper in its submission format is 58 pages: (as always click on the image to enlarge)
The published format will reduce the length a little (mainly shrinking the figures), but it will still be much longer than the standard 10-15 page paper.
One key figure to help explain some of these "lateral" concepts appear in Fig. 2, reproduced below. This figure shows a just-frozen droplet, which is often called a "droxtal". Crystal facets have appeared for eight faces, shown as the shaded flat regions in the top sketches.