The fun of shooting down your own theories
April 10th, 2014Thomas H. Huxley once wrote the famous line:
The great tragedy of Science: the slaying of a beautiful hypothesis by an ugly fact.
Great man, catchy phrase, but perhaps a bit overdramatic. To me, the slaying of a “hypothesis” (i.e., pet theory or just idea, really) can be the beautiful thing. It means that one can do a lot of damage with just a simple observation. I like it, even when I'm shooting down my own theory. Here's an example:
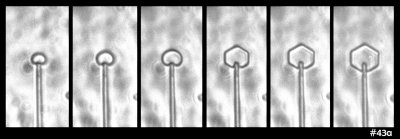
Some time ago in my experiments, I saw the following ice growth sequence:
What you see there is an extremely small, thin ice crystal growing from the tip of a glass capillary into air. (Size-wise, the glass capillary is about 5 micro-meters in diameter, or about 1/10th the thickness of the hair on your head.) I saw it happen several times. As the crystal grew, it developed the prism facets that generally define the hexagonal crystal shape. Other people had seen such rounded growth before, generally within a few degrees of zero (C), though in all cases, the crystal had been extremely thin. You can also see this thin, rounded (non-facetted) form in some hoar-frost formations.
The mystery here is why the disk grows without the prism facets for awhile. I never saw this with thicker crystals, so I formed a little theory. The theory involves the source of the water molecules to the curved region of crystal: some come from the vapor in the air and some wander over from the flat, non-growing crystal faces on front and back. In the 1960s, people had tried to measure this “wandering distance”, but never determined a consistent value. My theory predicted that once the crystal thickness exceeded about twice this distance, the curved edge would transition to flat, giving rise to the hexagon.
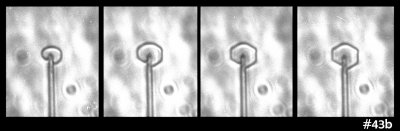
But then I looked closely at this case:
That sequence shows the side and front view. The crystal doesn't discernibly thicken when the flat prism facets appear. Zing! That theory shot down.
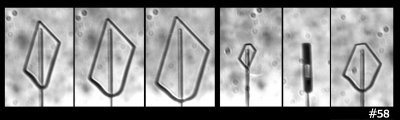
On to the next pet theory. So maybe the key factor is the diameter of the disk: One might argue that the curvature of the crystal surface must be below a certain value, which means a large enough diameter is needed, for the flat facets to appear. Or, the size of the resulting prism faces must be larger than that needed to have several surface steps (which help ensure flatness).
But then I look at this case:
In that case, I see both smaller and larger prism faces forming at the same time (same thing can be seen in the previous image). So, I guess the curvature or size of the resulting face is not the main factor. Zing!
Some researchers had observed slight bending of the prism facet above -2.0 C in equilibrium. They postulated a “roughening temperature” at -2.0 C. This might explain the rounded disc edge, but wait! 1) This disc edge becomes facet as it grows, so it is not merely temperature, and 2) these crystals are below -2.0 C.
Zing again!
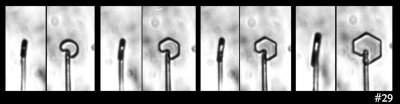
Well, there are always “impurities” to blame! Crystal growers are fond of blaming some trace, active chemical, or “impurity” for inexplicable results, so we could theorize that the above show the effect of surface impurities. As the crystal grows, the area over which the impurities distribute increases, thus diluting their concentration, and thus reducing their effect. Yes! This could explain these results.
But, wait, what about this:
The above shows two sequences (same capillary) in which some prism facets have formed, but some remain round. In the right-side case, one corner even rounds as it grows. Hmm, not a likely result of impurities. Zing!
So, I am down to one last theory. I do not yet have the data to shoot it down. And I'm not telling you, or I'd ruin the fun. We just need more data.
All in all, I think Huxley needs a little tweaking to serve my view:
The great beauty of science: the slaying of a pet idea by a simple observation.
-JN
Equinox frost I: Patterns on roof, hood, window, and mirror
March 24th, 2014Morning on the spring equinox, the first day of spring, brought a few gifts from winter. I) Film-frost, accentuated with hoar-frost on the cars.
See the white of the hoar, following a pattern set down by the thin layer of film-frost. The roof of one car:
And the roof of another:
So it went. As I walked through our parking area, I saw a different pattern on every car - a pattern that told a tale of the night's weather and evening conditions. The film frost here is dramatic because the water film was thick before it froze. And it was thick because it had been raining in the previous evening. Later that evening, the skies cleared, and the temperature dropped rapidly, ensuring that the thick film would freeze. And the warm wet weather of the previous day left plenty of vapor to deposit as hoar-frost.
Car hoods had their own story to tell:
Video of growing ice spike
March 6th, 2014Take about a cup of water, place in a glass flask, then plunge in liquid nitrogen. Watch.
">

What happens in that video is that the water starts to freeze from the bottom and sides of the flask, and the ice also spreads across the top surface. When the water turns to ice, it expands by about 10% and so the remaining water must be pushed aside. If a hole remains in the top ice layer, the water gets pushed out the top.
What happens next depends a lot on various, poorly studied features of the situation. But sometimes, the out-flowing water forms an ice spike like that shown above. Sometimes it doesn't. In a previous post (02/06/2011), I describe a vase-like shape that formed in an outdoor bathtub:
http://www.storyofsnow.com/admin.php?ctrl=items&blog=1&p=128
The trick of putting the flask in liquid nitrogen (about -200 C) came from Larry Wilen back in about 1989 when we were at the University of Washington. I later did the trick a few times at the University of Arizona, but I thought I'd try to perform it during a talk I gave in January, 2013. So, I went to see the chemistry-department demonstration guy, Eric Camp, at the UW, and we tried to replicate the demo. Now, in the past, I could get the spike pretty much every freeze, but Eric and I had the hardest time with it. So, in the end, I got the video, but didn't attempt the stunt at my talk.
- JN
The end of snow
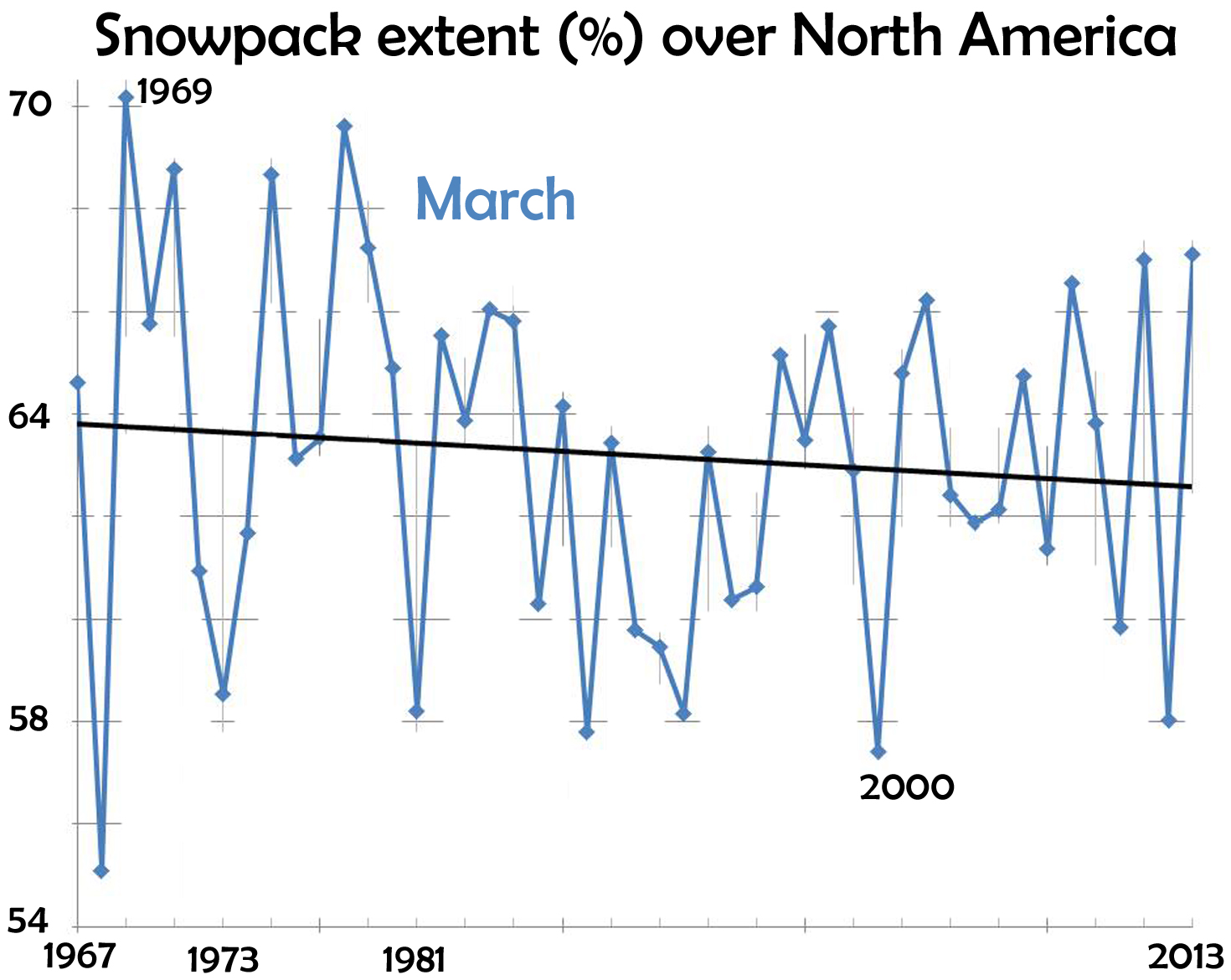
March 1st, 2014This recent front-page article caught my eye:
The writer is an avid skier-snowboarder, and thus concerned about the future of his sport. The facts he relates paints a grim picture:
- In the past 47 years, a million square miles of spring snowcover has disappeared from the Northern Hemisphere.
- Since 1970, the winter warming-rate has been triple the rate of the previous 75 years.
- The Alps are warming 2-3 times faster than the world-wide average.
- Potential Winter Olympic venues shrinks from 19 (now) to 6 by 2100.
and many other facts. Avid skiers like spring skiing. For March, the data (from Rutgers University Global Snow Lab) show a decline in area covered by snow.
The cup and the butterfly
February 25th, 2014In early January, while visiting a cold, dry region, I saw this frost on a wooden fencepost.
The pattern resembled a cluster of butterflies. In the shade, these "butterflies" were blue, reflecting the blue sky. In the sun, they were bright white:
These are a type of hoar-frost, and though hoar-frost grows by the same processes as snow crystals, they can take on an even greater variety of forms. That they have more forms is a consequence of the fact that they can experience much greater levels of humidity, that is humidity relative to their temperature. This greater degree of humidity produces faster growth. Frost forms can also be more unusual because the proximity of the crystals alter the vapor gradients.
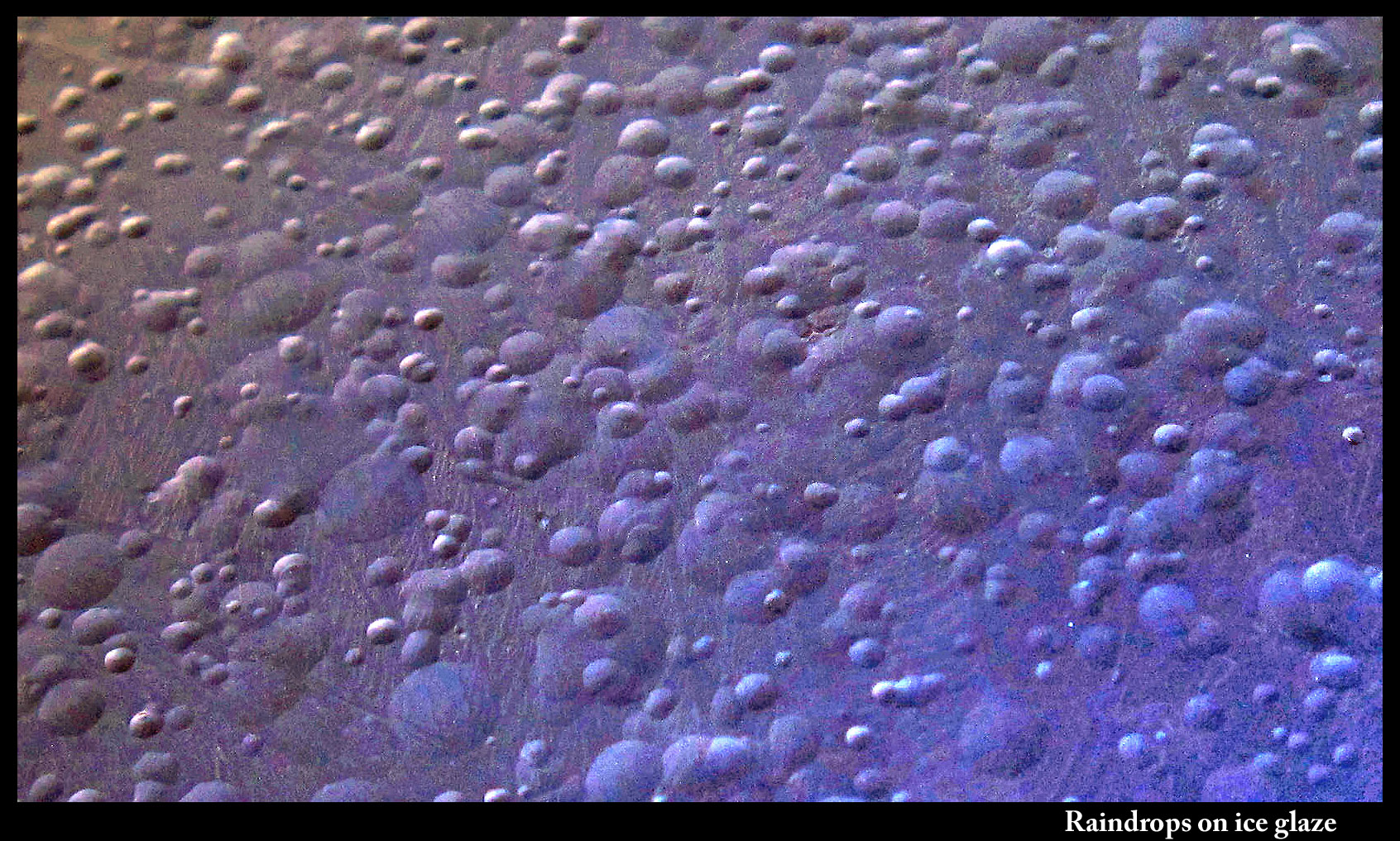
Raindrops on ice
February 23rd, 2014When water droplets land on ice, what happens?
If the ice is at least several degrees below zero (celcius), the drops freeze quickly, and build up a whitish, bumpy surface.
When the ice surface is heated to melting, the droplets vanish into the melt.
When the ice is instead very close to zero, the droplet spreads out, but not completely. You can still see the boundary of the droplet.
In the early 90s, there was some scientific debate about the ice surface near zero. Some said it had a thin, liquid-water surface, a layer that allowed us to ice-skate and make snowballs. Others said that if that were the case, then a droplet placed on the ice would spread out and vanish. Experiments showed the droplets didn't vanish. The jury is still out on the nature of the ice surface.
-JN
Bending of branch and pond
February 11th, 2014Bending bending bending
The fir branches are bending
They are waiting for more snow
The famous Japanese poet Basho wrote a poem like the above, except it was about bamboo bending under the snow load.
I was reminded of Basho's poem yesterday, as we just had our first snowfall of the season. Here in Redmond, about 2 inches, just enough to bend some of the fir branches.
And not only the branches were bending.
I took the opportunity to check out the pond. It had an ice covering before the snow, but with the snow and warming trend, the ice had thinned, and gotten pressed down under the snow load. The glaze over the pond was bending. Where a hole appeared in the ice, water got pushed up and out, flowing in dark channels over the icy glaze, forming a spider-like or branch-like pattern in the now slushy snow. I climbed up a tree on the shore and took a few shots.
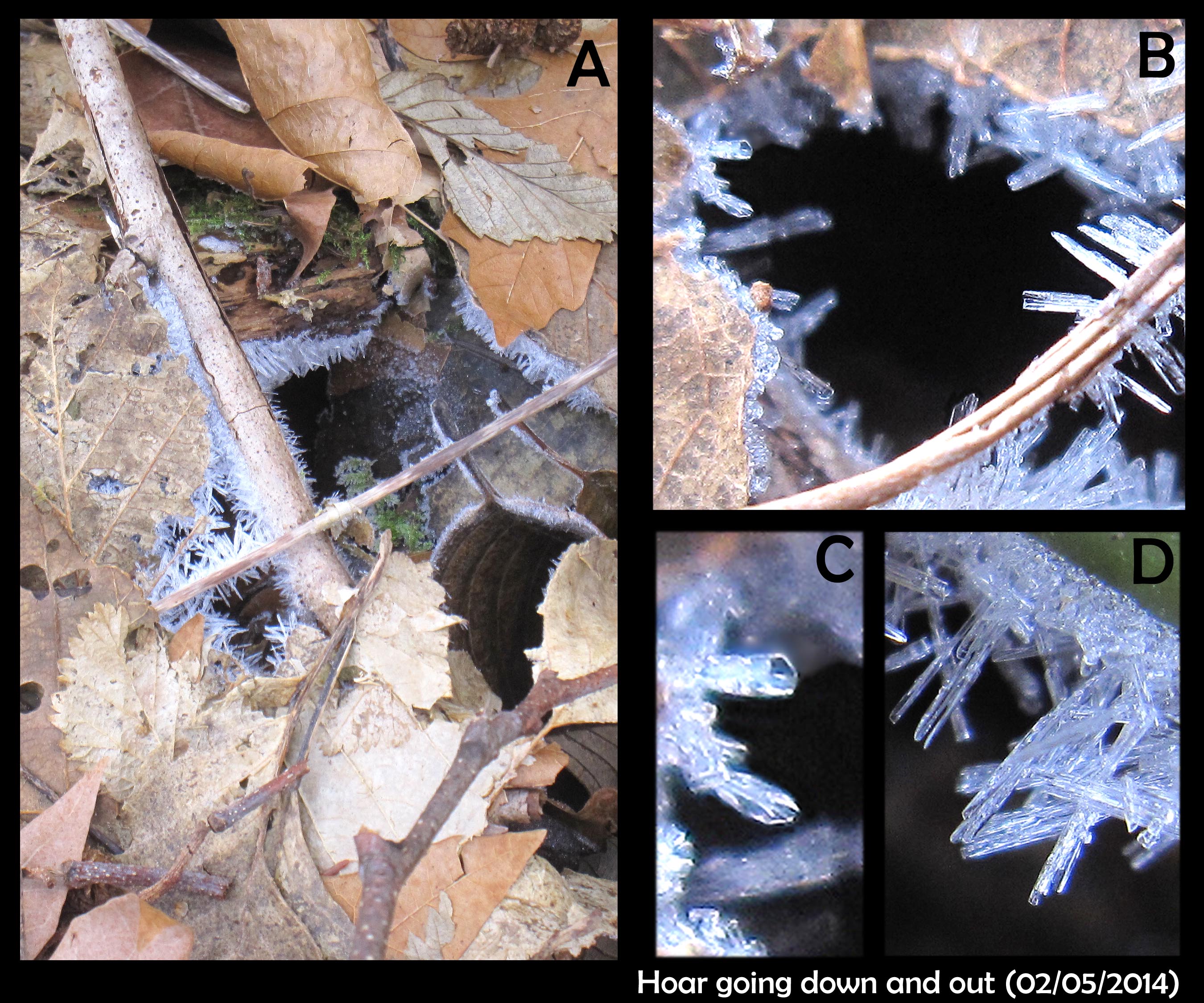
Hoar that grows down and out
February 9th, 2014It had been a continued spell of cold days and nights, the ground snow-free, the air clear and dry. No film-frost on the cars in the morning, and no spikes of hoar frost sticking up on grass or post. The only signs of ice had been the frozen pond and the needle ice, making the ground crunchy underfoot.
And yet in the woods and lawn, hoar frost still lurked.
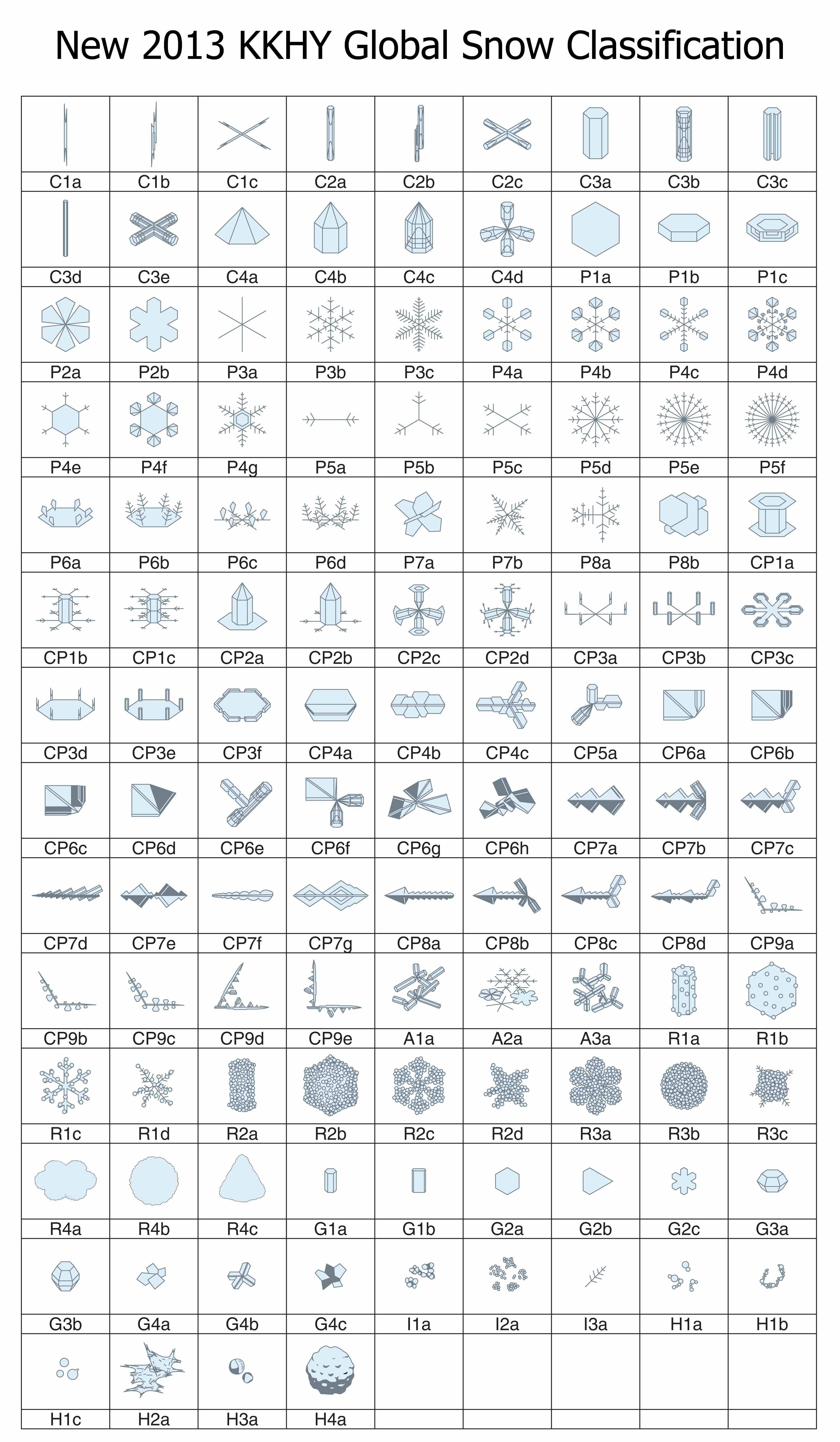
One hundred twenty one forms of falling ice: the new snow classification system
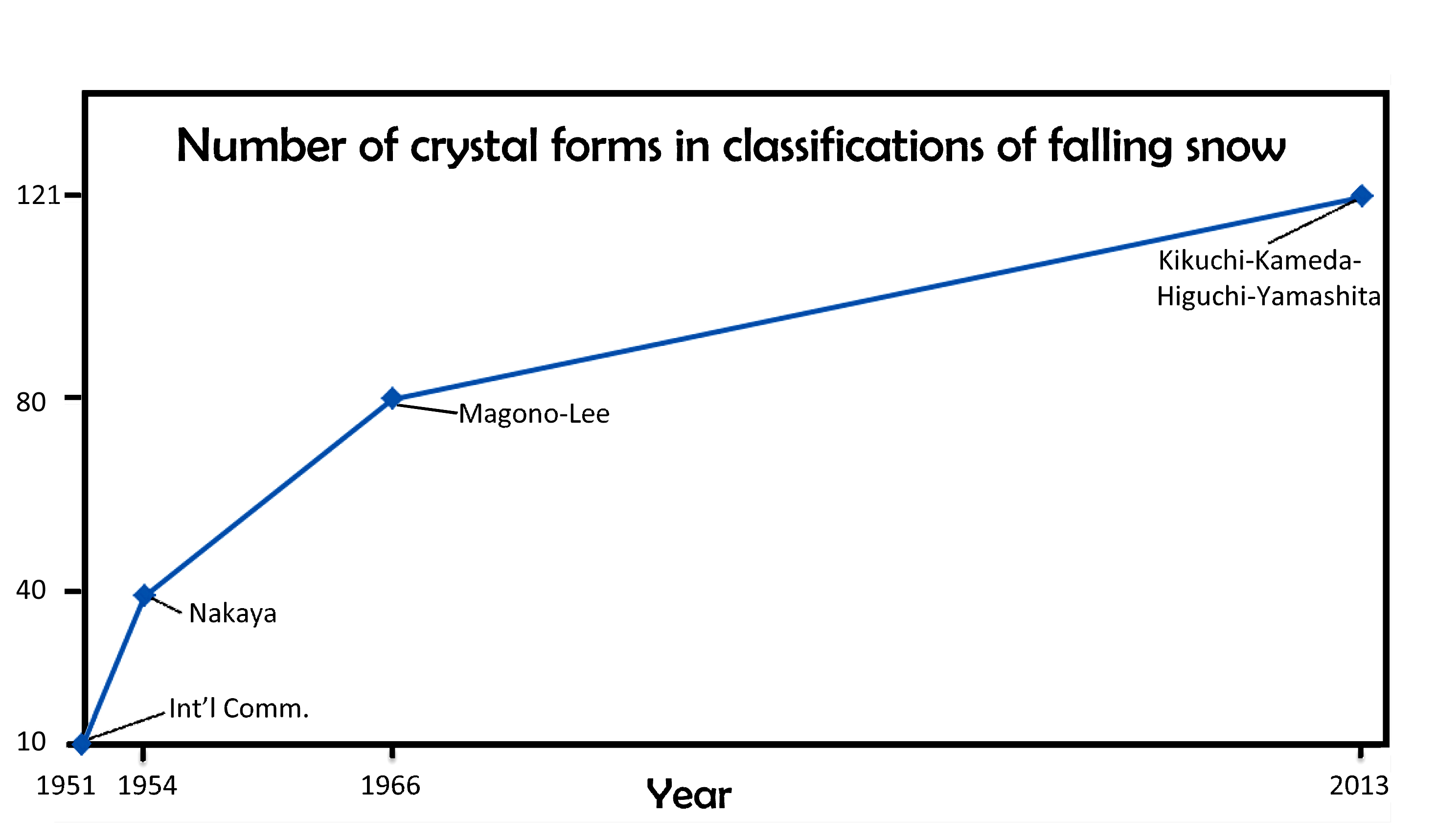
January 22nd, 2014When I last wrote about the classification of falling snow and ice (02/14/2010), I discussed the 1966 Magono-Lee system. At the time, this system was the most recent one, and as such, the one generally used in meteorology. And to those who wondered how many types of icy precipitation exist, Magono and Lee would tell you 80 types.
Although 80 sounds like a lot, Magono and Lee did not include at least one very common type as well as a few other interesting types. This major omission was snow-crystal aggregates, generally known as “snowflakes” by meteorologists. These are individual snow crystals that have stuck together during their fall and arrive as large open clusters of crystals, often with tens–to–hundreds of crystals in a big, round blob. (To meteorologists, a snow crystal is not a snowflake; rather, a snowflake consists of many snow crystals. It is like the difference between a trundled rock and a landslide.) In fact, the snowflake is probably the most common type of snow precipitation in most areas that receive snow. Several other omissions of the Magono-Lee that I mentioned in my 2010 posting included spearheads, seagulls, and the both the 18- and 24-branched forms.
If you were waiting for someone to fix the classification, well wait no more: we now have a new classification scheme that includes all of these and more. The new system has not one, but three types of snow-crystal aggregate (snowflakes), which are given the symbol “A” (for aggregate). There is also four types of spearhead crystal (symbol “CP8”), five types of seagull crystal (symbol “CP9”), and both the 18- and 24-branched crystals (P5e and P5f). In all, the new system has added 41 new types. In one big graphic, here is the new system:
The architects of this new classification scheme published the above table in summer 2013*. There were four authors (K. Kikuchi, T. Kameda, K. Higuchi, and A. Yamashita), making it harder to name the system after the originators, but hopefully their names will become known, just as Magono and Lee’s have.
I will discuss various aspects of this new system in subsequent postings. But for now, I point out that the number of basic types has increased over the years, from 10, to 40, to 80, and now 121. When will it ever end?
Of course, it may just keep increasing. In fact, if we plot the numbers vs the years, and look at the pattern, we might conclude “never”.
2013 Snow Crystal Photos
February 8th, 2013
In the last few weeks, the winter of 2012/13 has been pretty good for snow crystal photos. Below is one sample, and you can see a full set of this season's photos on my flickr account -
http://www.flickr.com/photos/markcassino/8456495579/in/set-72157632724561546/lightbox/