Category: "Snow Science"
Dark lines in melting snowpiles
March 30th, 2019Snow cleared from a city street slowly vanishes, leaving lines of "dirt".
(Click on images to view more closely.) Where the snow has a distinct edge, you can see the dark lines are ridges, some of which can be quite sharp:
Why is this?
Cryoconite ridging
This all happens because the snow is vanishing but the dirt is not. The vanishing is actually called "ablation", meaning some combination of melting, sublimating, and evaporating. It is mostly melting in the above case, but it is possible that evaporation helps to form the lines. About the dirt, it is not clear exactly what it is. The term "cryoconite" is used when similar dark dirt falls and clusters on glaciers and ice sheets, so to be specific, we might call it "cryoconite ridging".
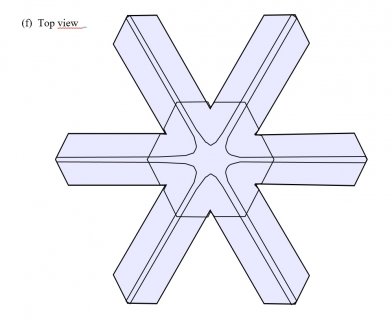
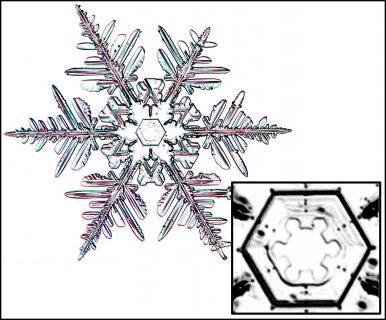
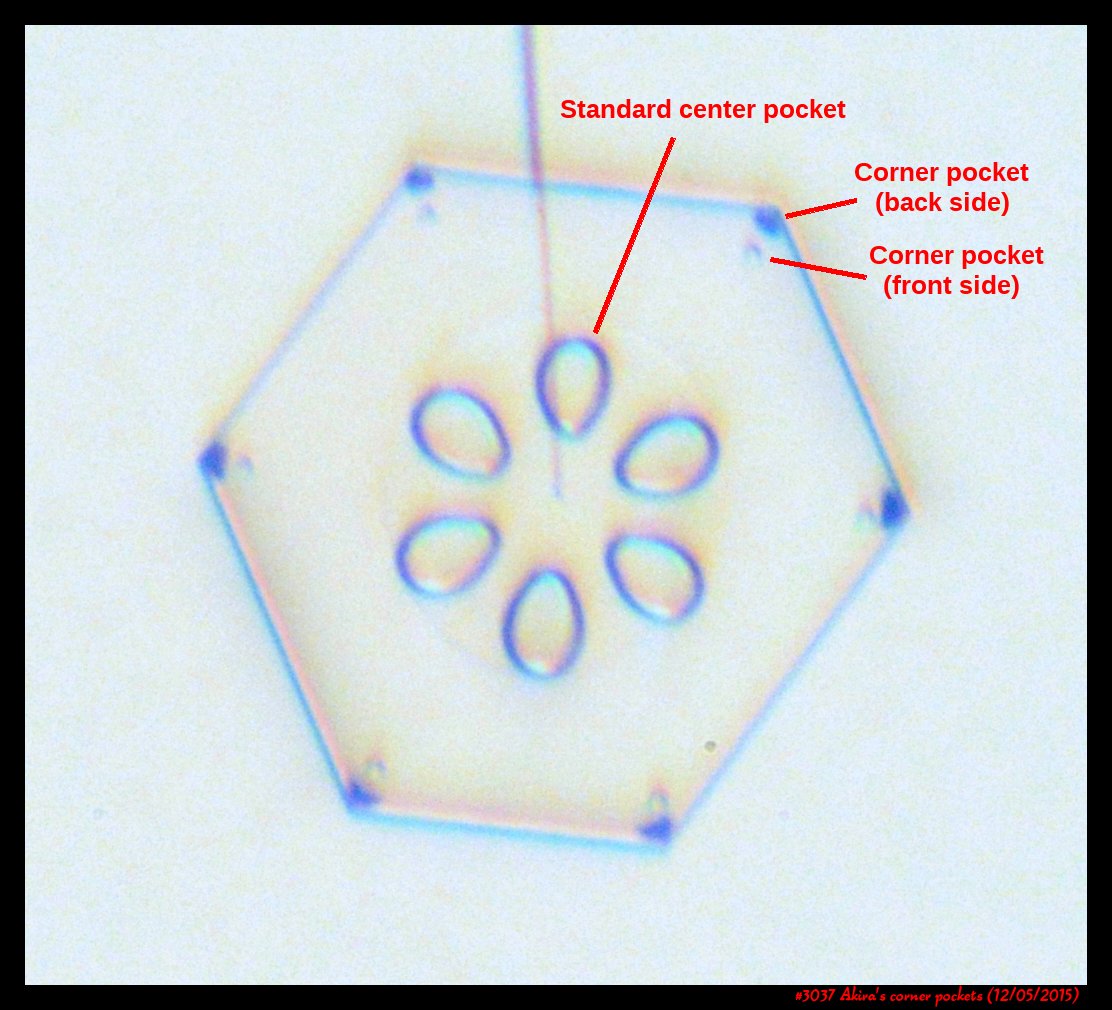
Poster on corner pockets in snow crystals
February 18th, 2019Last July, I attended the AMS (American Meteorological Society) 15th Conference on Cloud Physics, which was combined with a similar conference on atmospheric radiation. They have these about every four years, but I have missed all those going back to 1995. So, I had a lot to catch up on. Actually, by far the most enjoyable part of this conference was meeting old colleagues and making new acquaintances, including many who I had known only through their papers and email correspondence. As to why I waited so long, I suppose the main reasons were time and cost. Even without travel and lodging expenses, conferences are expensive. This one was $600, and the fee for the abstract I submitted was an additional $95. But in this case, the conference was only a 3-hr drive away (Vancouver, BC) and my co-worker very nicely picked up the conference fee and hotel tab. Also, this conference had a special session dedicated to the work of my former advisor, Prof. Marcia Baker. In that session, I presented the following poster.
I've described a little about corner pockets in a previous post, and showed a few of these panels before, but will briefly go over them here.
Bad Snow
November 28th, 2017This post is about the misrepresenting of snow crystals in public, not about misbehaving crystals and not about snow that has gotten dirty.
No doubt you've seen it, the Christmas card with four-pointed "snow" falling, or the sweater with an eight-pointed "snow" emblem. Once, at a holiday party, I saw such a sweater and remarked on it to the wearer. I was told that, with snow, "no two are alike", and apparently that was supposed to justify anything goes. Sorry, but it doesn't work that way. After all, we can say the same thing about people, that is, no two of us are exactly alike, and yet we do not regularly see sketches of people with five arms or three heads. That is because people do not come in these shapes. Similarly, snow-crystal growth allows unlimited crystal forms, but nevertheless follows strict rules. If you understand these rules, you too can point out impossible snow-crystal shapes. I give the rules below.
But to help illustrate these rules, I first present below examples of real and "good" snow, together with some examples of bad snow:
Click on this (and any image here) to see a larger version.
Columns!
January 18th, 2017The poor columns get left out of nearly all snow-crystal discussions, but they are an interesting type. So, to help them out a bit, here's my first column appreciation post.
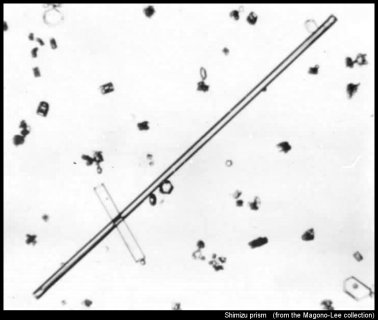
Let's start with perhaps the most extreme column of all, the Shimizu prism*:
I say 'extreme' because they are so long and thin--sometimes over 1-mm long yet just 0.01-0.02 mm in diameter. These types have so far been found to fall only on the Antarctic Plateau. But in theory, they should be able to form elsewhere. It is like a "whisker" crystal, which Teisaku Kobayashi grew below -50 C on a surface in the lab. The image above shows many other crystals as well, including another solid column crossing the Shimizu prism.
Next, the bullet rosette:
The bullet rosette is most often found below -25 C in high cirrus clouds. It is an example of a polycrystal; in this case, a frozen droplet that froze into several distinct crystals (one for each "bullet").
Next, one of my favorites, the scroll column (though the picture doesn't quite do it justice):
In this form, the sides of the crystal seem to fold inward, like a scroll.
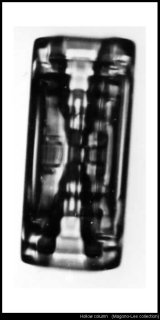
Finally (for now, anyway), the ubiquitous hollow column:
The funny banding you see (horizontal lines inside the 'hollow') is a mystery.
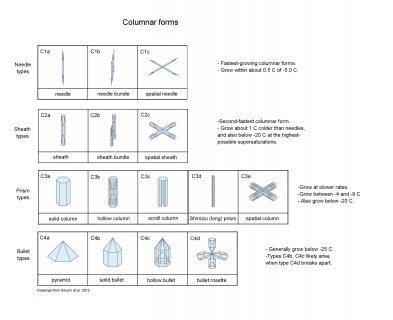
There are many other columnar forms, many of which are in the following diagram (as with all images here, click on it to see it enlarged)**:
One neat thing about the columnar forms is that you can see roughly exact replicas of them in hoarfrost. The Shimizu prism may be hard to find, but the others are common if you look closely.
-JN
* Images are from the Magono & Lee collection, used in their paper: Meteorological classification of natural snow crystals. J. Fac. Sci., Hokkaido Univ., Ser. VII 4, 321–335.
**Drawings are based on those in Kikuchi, Kameda, Higuchi, and Yamashita: A global classification of snow crystals, ice crystals, and solid precipitation based on observations from middle latitudes to polar regions. Atmos. Res. 132-133 (2013) 460-472.
How clouds form snow
January 14th, 2017To understand snow formation, one must know a little about clouds.
Q: What is in a cloud?
A: Air, dust, vapor, droplets, and often, ice.
Q: How much air? How much liquid water? How much ice?
A: The answers will probably surprise you. See my short 20-min presentation below. I gave this recently to the Bellingham, WA Snow School. (23 slides, but due to file-upload-size restrictions, I had to put them into three parts below, 10 slides, 6 slides, 7 slides.)
Snow, rain, and weather affect everybody, yet how many of us learned in school even the most basic facts about precipitation in school?
Q: Who first realized how ice grew in a cloud?
As described in my presentation, he realized this by observing frost on the ground.
Q: Who first realized how Alfred's theory was intimately connected with rainfall?
Tor discovered this by observing fog in a mountain forest, and like Alfred, applied some of his physics knowledge.
In my presentation, I discussed Alfred Wegener, the roles of the different cloud components, and briefly how the ice, once formed, takes on its strange shapes:
First 10 slides (with blue text added to account for the things I said during the talk):
http://www.storyofsnow.com/media/blogs/a/Jan2017/snowschool_annotated1t10.pdf?mtime=1484585328
Next 6 slides:
http://www.storyofsnow.com/media/blogs/a/Jan2017/snowschool_annotated11t16.pdf?mtime=1484585328
Last 7 slides:
http://www.storyofsnow.com/media/blogs/a/Jan2017/snowschool_annotated17t23.pdf?mtime=1484585309
Later, I will show specifically how the ice gets arranged into all these strange shapes.
- JN
Snow Science: an annotated list to topics
December 17th, 2016For those interested in the science of snow, I give an annotated list of relevant blog links.
I) Seminar about snow science (sequence of four videos):
http://www.storyofsnow.com/blog1.php/trip-of-the-ice-man
II) All the different types of falling ice in one diagram:
An older, simpler diagram:
http://www.storyofsnow.com/blog1.php/how-to-classify-snow-crystals
III) Why snow has six sides (the real answer, not the sloppy, standard answer):
http://www.storyofsnow.com/blog1.php/how-the-crystal-got-its-six
IV) How snow forms and how it is analogous to people:
http://www.storyofsnow.com/blog1.php/i-am-a-giant-snow-crystal-imperfect-thing-of-habit-bouncing-along-life-s-gusts
V) How to determine what the cloud conditions were like by looking at a snow crystal:
http://www.storyofsnow.com/blog1.php/new-habit-diagram-for-branched-tabular-crystals
VI) How snow crystal shapes are like frost crystal shapes (many blog entries about this, but here are just two):
http://www.storyofsnow.com/blog1.php/a-change-in-habit
http://www.storyofsnow.com/blog1.php/the-cup-and-the-butterfly
VII) Why is snow sometimes white and sometimes black? Also, why is snowpack so bright?
http://www.storyofsnow.com/blog1.php/white-snow-black-snow-pink-snow-blue-snow
VIII) Some finer details of those star-shaped crystals and what they tell us:
Two-level;
http://www.storyofsnow.com/blog1.php/two-level-nature-of-branched
Droplet origin:
http://www.storyofsnow.com/blog1.php/how-some-snow-crystals-hide
http://www.storyofsnow.com/blog1.php/how-some-snow-crystal-centers
A signature of sublimation:
http://www.storyofsnow.com/blog1.php/how
http://www.storyofsnow.com/blog1.php/akira-s-corner-pockets
IX) If you look up at a cloud, how do you know it has ice crystals in it?
http://www.storyofsnow.com/blog1.php/it-s-not-a-rainbow-it-s-better
http://www.storyofsnow.com/blog1.php/halo-in-the-sky-uh-i-don-t-see-no-halo
Over the coming year, I'll be adding to this and reposting it.
-- JN
Two-level nature of branched crystals
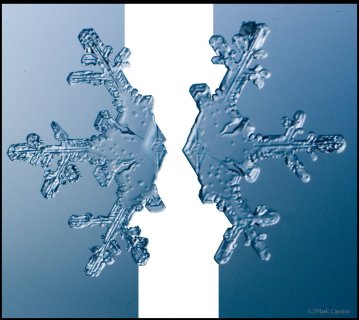
December 11th, 2016The common branched crystal looks like a paper cut-out, but actually has a complex 3-D nature. One aspect of this nature, which I have alluded to in prior posts, is the two-level structure: What appears to be happening on one plane, is actually occurring on two. For example, consider the following dendrite of Mark's:
The six branches of this crystal are not on the same level: the three on the right are on the top level, whereas the three on the left are on the bottom. If I could break the connection between the levels and pull them apart, they would look similar to this:
Actually, the connection can be broken. The fellow who first did this was Ukichiro Nakaya back in the 1930s.
Instead of using photoshop (as I did above), Nakaya (or rather Nakaya's assistant) used a razor blade in a very cold room. His assistant must have been both highly skilled as well as very patient, because each level is separated by less than half the width of a typical human scalp hair.
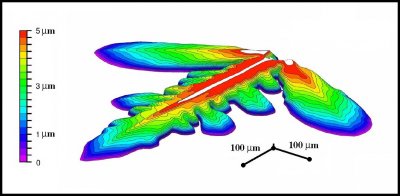
The levels themselves are even thinner. For a fern-like dendrite, which may be 2 mm across, the thickness of one level may be 300 to 500 times thinner. A recent study ** by Wataru Shimada and Kazuki Ohtake of Toyama Univ. in Japan used a laser-imaging device to map out the contours on such a branch. The result shows an intricate 3-D structure:
(Thanks to Prof. Shimada for sending me this image. I have slightly simplified it.) The image shows the central part of the branch as a ridge, with numerous valleys running down both sides. The thickness of the region in red is only 5 micro-meters (5 millionth of a meter or about 0.2 thousandths of an inch).
None of this is the flat facet that we imagine a snow crystal to be. There is one flat facet however -- it is on the bottom side.
-- JN
**The study is here, though not freely accessible: http://pubs.acs.org/doi/abs/10.1021/acs.cgd.6b01263
How some snow crystals hide their droplet origin
December 5th, 2016Look closely at the center of a snow crystal. In many, or most, you will often not find a droplet center as we described in the previous post. Indeed, for the columnar crystals, you may never see a droplet center. As an example, look at the center of the dendrite crystal below (one of Mark's):
Why? Do these not also start on droplets?Or are the droplets just too small?
This crystal, like all snow crystals did start on a frozen droplet, but the vapor deposited all around the frozen droplet (also called a 'droxtal'). The resulting growth can vary quite a bit, but roughly proceeds in a way first described in detail by the physicist Sir Charles Frank:
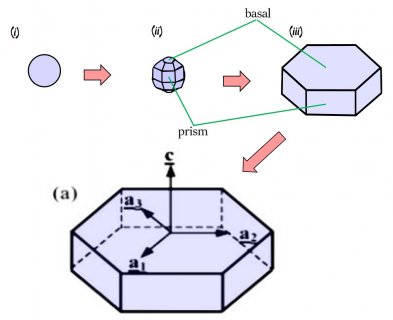
The crystal will still develop its two levels, top and bottom, just like we saw in the previous post, but in this process, it takes a little longer for the two levels to develop. The first stages involve the frozen droplet gaining flat faces (see steps (i) to (ii) below). Initially, as in (ii), there are more than 8 faces, but the fastest-growing faces soon vanish (they essentially grow themselves out of existence), leaving only the slower basal and prism faces in (iii):
The slower-growing faces win the game and are allowed to reveal themselves. With snow, we learn that sometimes slow wins.
In the above sequence, follow the red arrows for the time sequence. (From "(a)", I am borrowing some sketches I made for a publication in 2008. These differ slightly from those originally proposed by Frank.) The crystal sizes here are very small, about 1/10 to 1/2 the width of typical scalp hair (i.e., about 7 to 40 micro-meters). The axes on "(a)" show the crystallographic axes for ice, remnants of my paper.
You can see though that by stage (iii), the form of the original droplet has vanished. But let's continue, along the path to a recognizable branched crystal, roughly as described by Frank.
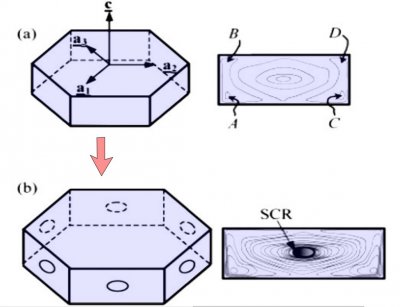
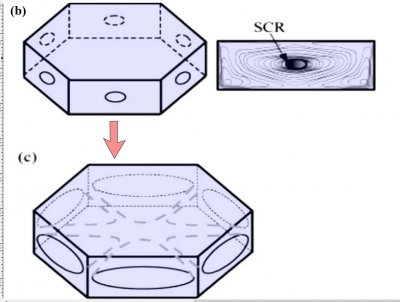
The crystal face grows outward by the spreading of layers. In the sketch above, the edge of these layers, called "steps", are shown by fine lines in the front view at right. The steps start near a corner, say at "A" or "C" in the sketch, and spread inward, closing in on the center. But soon a problem emerges: the steps are coming too fast and the center region cannot keep up. So a small pit emerges in the center of the face as you see at (b). On a symmetric hexagonal form like that above, this pit forms roughly at the same time on all six "prism" faces. (Pits may also form on the top and bottom faces, but for a crystal shape like that above, will not become very deep.) Here "SCR" stands for step-clumping region.
Typically, the rim of this pit expands as the crystal grows (c).
And grows. Eventually, the rim breaks through a crystal edge. Usually, it is the edge between two prism faces as shown below.
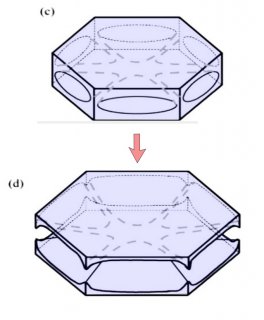
Notice that at stage (d), the crystal now has two levels, a top side and a bottom side. From this stage on, the two levels will compete with each other, with one eventually getting much larger than the other. This same process was shown in the previous post. But let's continue on and see how this develops.
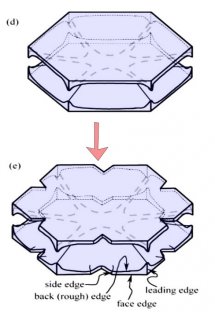
Either one, or both levels may sprout branches. In the case (e) above, both have sprouted (note the notches that divide the just-sprouted branches). So far, both levels are developing at the same rate. But that cannot continue: the situation is unstable because if one level gets just a little bit ahead, it will stay ahead and increase its size difference with time. In fact, the smaller level will hardly grow at all - it is stuck in a region largely devoid of vapor excess.
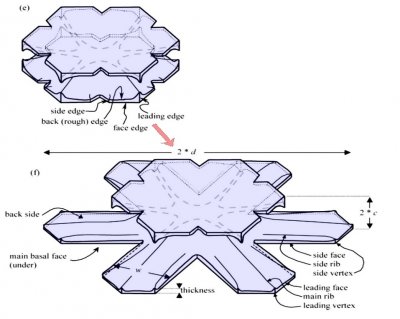
Typically, it is the bottom level that gets ahead, as shown in (f) above because the crystal is falling down through the onrushing vapor-laden air. (The interior lines labeled in (f) above will be discussed another day.) Looking directly down on this crystal, it would look roughly like the following.
Consider that every crystal is a little bit different, but the basic features in (f) exist in the original picture at the top. I reproduce it below with a close-up of the center.
Some of these finer details will be discussed in a later post.
Finally, as to why this process sometimes occurs but sometimes the process of the previous post occurs instead remains a mystery. There are a few factors that will favor this process though: smaller initial droplets, and conditions of slower growth.
-- JN
How the water molecules make corner pockets
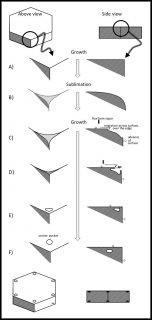
November 11th, 2016As I mentioned in my previous post, the formation of corner pockets on ice crystals is inexplicable by the standard theory of snow-crystal growth. Akira Yamashita had recently proposed a mechanism for corner pockets, though he had applied it to a different situation. In his case, he observed the early growth stage of a relatively large frozen droplet as it transitions into a faceted crystal. So, his situation dealt with a round crystal becoming sharp-edged. In our case, we started with a large sharp-edged crystal, then made it become round by sublimating (analogous to evaporation, but for a crystal), and finally made the crystal re-sharpen by growing it. The process is sketched below. You may want to open the image in another window and enlarge it.
At the top, you see the simple hexagonal prism crystal in its initial stage. At stage B, the crystal sublimates (we reduced the amount of vapor), meaning that it is starting to shrink. The shrinking starts from the corners, making them become rounded. Then, in stage C, we start growing the crystal again, and the interesting things start to happen.
As the water molecules from the vapor stick to the surface, they remain mobile on the surface. On the surface, they wander around until latching into a little nook. (In crystal-growth theory, these 'nooks' are actually tiny kinks in a surface step.) The region just over the edge has plenty of nooks, as it is rounded. So, the edge grows fast and starts to protrude and overhang (stage D). Overhangs from the top and side faces merge in stage E, creating the pocket. Afterwards, water molecules in the pocket surface move about making a more round, disk shape.
About being inexplicable by the standard theory of snow-crystal growth, this standard theory does not include the wandering of the mobile surface molecules over the edge. Now, if the new theory applied only to this corner-pocket formation, then it would be of little importance. But the wandering process cannot apply to only this situation (how would the molecules know when to start or stop?): It must ALWAYS be happening. And, as I mentioned earlier, I realized that the wandering process may help explain many other curious snow-crystal features that I had previously found inexplicable. In other words, it may be a fairly important discovery.
-- JN
Akira's Corner Pockets
May 10th, 2016The evanescent snow crystal
appears out of nowhere
The lines and boundaries
on its faces record a story
a story of a crystal's birth
a story of a crystal's life
But before the record vanishes
Who will hear its story?
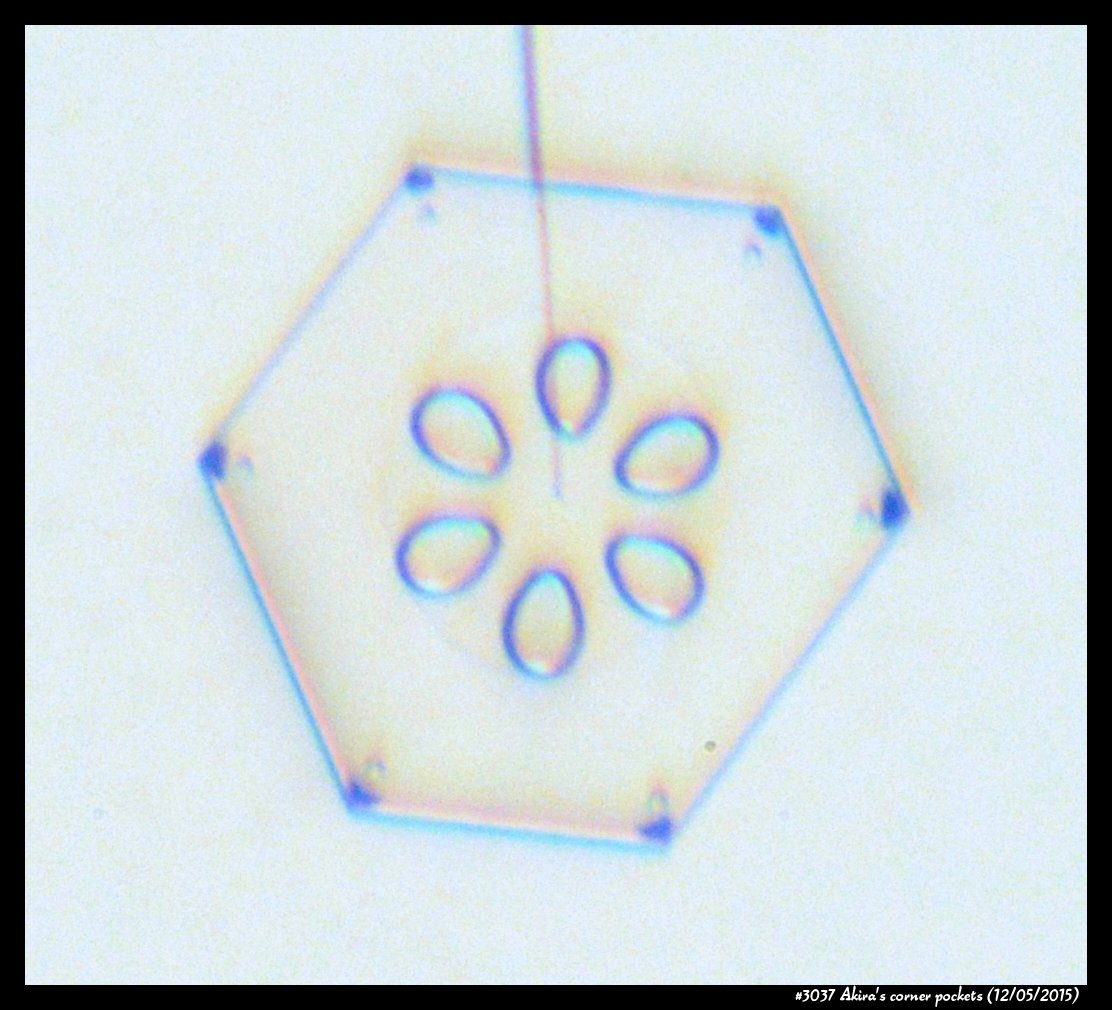
A few years back, a correspondent of mine, Professor Akira Yamashita of Japan, long retired, sends me an email. In the email, he had a document with words and pictures of some small crystals that he'd captured back in the 70s. They were small crystals, essentially freshly "hatched eggs" from the frozen droplets upon from they had started. But some had small pockets of air near their corners.
To those who have studied any sort of crystal growth and have some familiarity with crystal-growth theory, these corner air pockets, or "bubbles", were in impossible locations. They should not be there. Pockets will form near face centers, not corners. But Prof. Yamashita also had a theory about their formation. His theory first looked sketchy to me, but I appreciate hearing about new ideas, so over the following years kept revisiting his theory, getting to think that it had merit, and wondering if it had other applications.
Then, just this past year, in our own ice-crystal experiments, we did something that apparently had never been done to small ice crystals in the lab before. We slowly grew a crystal in air. And we cycled it from slightly growing, to slightly sublimating (i.e., shrinking in size), to slightly growing again. A cycle that must happen in some regions of cloud. And here is what we saw:
Corner pockets!
After the sublimating, the subsequent growth kept a permanent record of the sublimation cycle in the form of 12 corner pockets, one pocket for each of the 12 corners of the crystal. These are pockets of air, just like the six large 'petal-shaped' pockets of air you see nearer the center of the crystal. They are forever stuck in the crystal. Stuck there until the crystal, with all of its features, vanishes back to air.
After seeing this, we ran a few more experiments, and each time we slowly grew, then sublimated, then grew again, we got corner pockets. The name 'corner pockets' refers to their location when they are formed; namely at the corners, next to the crystal perimeter. However, they remain essentially fixed in position as the crystal grows, and this means that as the crystal perimeter expands outward, the corner pockets will appear further within the crystal. Analogously, the 'center pockets' shown above formed at the face centers, on the crystal perimeter, back when the crystal was much smaller.
As to the theory of their formation, and how the theory can explain other observable features of snow crystals, you'll have to wait for a subsequent post.
- Jon