Category: "Announcements"
New paperback edition out, Sept. 2017
October 25th, 2017After eight years, several translated editions, a teacher's guide, and a Scholastic edition, The Story of Snow had a paperback edition, also from Chronicle books. The size is the same, and the content is the same except for one thing. This version lists some of the glowing reviews and accolades on the inside cover (click on image to read):
- JN
A complete picture book of all (known) snow types
February 24th, 2017Prof. Katsuhiro Kikuchi, a highly regarded ice and snow researcher from Hokkaido, Japan, kindly mailed me a copy of his wonderful new book (coauthored with Dr. Masahiro Kajikawa). Never before has there been anything nearly as complete as this book in describing and showing all known types of falling snow. It is called "Picture Book of Natural Snow Crystals” and published by the Hokkaido Shinbun News Paper Co., Sapporo.
The only issue for most of the readers here is the fact that it is written in Japanese. This issue though does not mean one cannot learn a lot from the pictures. Here are a few sample pages:
First, the broad-branch planar crystals P4d, P4e, and P4f:
Next, some basic combination forms (columnar and planar), specifically CP1a and CP1b:
A few of the above have been named after the Japanese-type drum "tsuzumi" due to their resemblance to this drum. Next, a type named for its resemblance to a flying seagull, the CP9a and CP9b:
Finally, a sample of the many explanatory diagrams:
The above diagrams explain how some crystals transform from a frozen sphere ("droxtal"), which all snow-crystals begin as (excluding broken fragments), to the larger forms we see on the pages. About these crystal images, Prof. Kikuchi collected them from a range of locations he visited including his nearby area of Hokkaido and Honshu, and further north and south, to the Arctic, Greenland, and the South Pole. So, though it may be possible, don't expect to see all these forms in your neighborhood.
Overall, it is an amazing book, and I wish we had something like it in English.
- JN
How some snow crystals hide their droplet origin
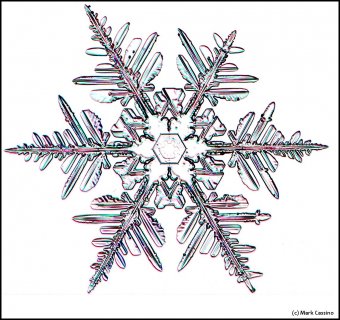
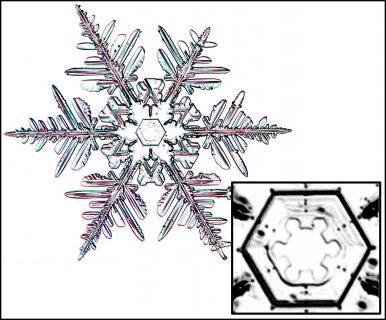
December 5th, 2016Look closely at the center of a snow crystal. In many, or most, you will often not find a droplet center as we described in the previous post. Indeed, for the columnar crystals, you may never see a droplet center. As an example, look at the center of the dendrite crystal below (one of Mark's):
Why? Do these not also start on droplets?Or are the droplets just too small?
This crystal, like all snow crystals did start on a frozen droplet, but the vapor deposited all around the frozen droplet (also called a 'droxtal'). The resulting growth can vary quite a bit, but roughly proceeds in a way first described in detail by the physicist Sir Charles Frank:
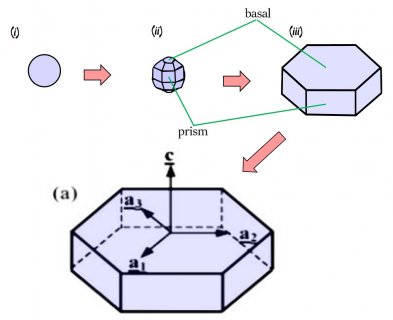
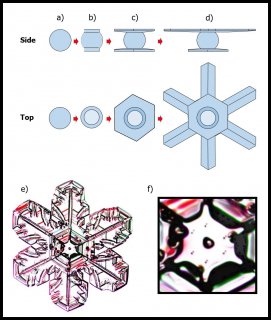
The crystal will still develop its two levels, top and bottom, just like we saw in the previous post, but in this process, it takes a little longer for the two levels to develop. The first stages involve the frozen droplet gaining flat faces (see steps (i) to (ii) below). Initially, as in (ii), there are more than 8 faces, but the fastest-growing faces soon vanish (they essentially grow themselves out of existence), leaving only the slower basal and prism faces in (iii):
The slower-growing faces win the game and are allowed to reveal themselves. With snow, we learn that sometimes slow wins.
In the above sequence, follow the red arrows for the time sequence. (From "(a)", I am borrowing some sketches I made for a publication in 2008. These differ slightly from those originally proposed by Frank.) The crystal sizes here are very small, about 1/10 to 1/2 the width of typical scalp hair (i.e., about 7 to 40 micro-meters). The axes on "(a)" show the crystallographic axes for ice, remnants of my paper.
You can see though that by stage (iii), the form of the original droplet has vanished. But let's continue, along the path to a recognizable branched crystal, roughly as described by Frank.
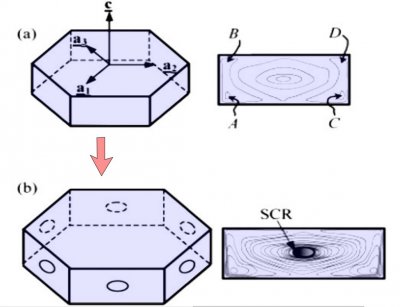
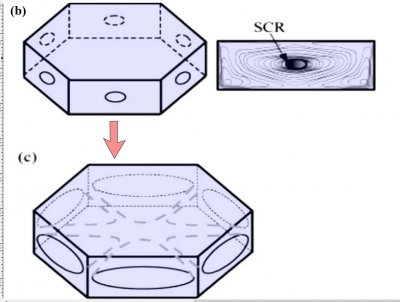
The crystal face grows outward by the spreading of layers. In the sketch above, the edge of these layers, called "steps", are shown by fine lines in the front view at right. The steps start near a corner, say at "A" or "C" in the sketch, and spread inward, closing in on the center. But soon a problem emerges: the steps are coming too fast and the center region cannot keep up. So a small pit emerges in the center of the face as you see at (b). On a symmetric hexagonal form like that above, this pit forms roughly at the same time on all six "prism" faces. (Pits may also form on the top and bottom faces, but for a crystal shape like that above, will not become very deep.) Here "SCR" stands for step-clumping region.
Typically, the rim of this pit expands as the crystal grows (c).
And grows. Eventually, the rim breaks through a crystal edge. Usually, it is the edge between two prism faces as shown below.
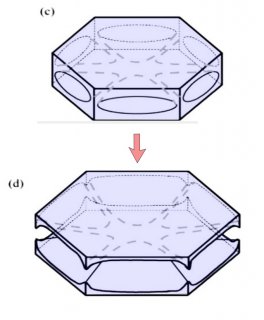
Notice that at stage (d), the crystal now has two levels, a top side and a bottom side. From this stage on, the two levels will compete with each other, with one eventually getting much larger than the other. This same process was shown in the previous post. But let's continue on and see how this develops.
Either one, or both levels may sprout branches. In the case (e) above, both have sprouted (note the notches that divide the just-sprouted branches). So far, both levels are developing at the same rate. But that cannot continue: the situation is unstable because if one level gets just a little bit ahead, it will stay ahead and increase its size difference with time. In fact, the smaller level will hardly grow at all - it is stuck in a region largely devoid of vapor excess.
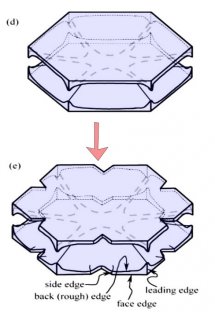
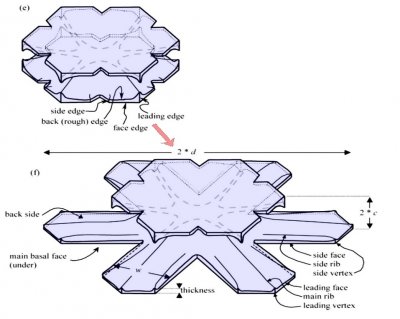
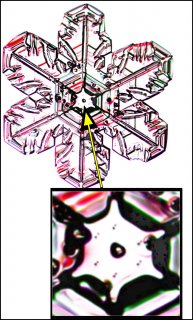
Typically, it is the bottom level that gets ahead, as shown in (f) above because the crystal is falling down through the onrushing vapor-laden air. (The interior lines labeled in (f) above will be discussed another day.) Looking directly down on this crystal, it would look roughly like the following.
Consider that every crystal is a little bit different, but the basic features in (f) exist in the original picture at the top. I reproduce it below with a close-up of the center.
Some of these finer details will be discussed in a later post.
Finally, as to why this process sometimes occurs but sometimes the process of the previous post occurs instead remains a mystery. There are a few factors that will favor this process though: smaller initial droplets, and conditions of slower growth.
-- JN
How some snow-crystal centers retain their droplet origin
November 22nd, 2016If you zoom in on the center of some snow crystals, you may see a small circle, or near circle. In some cases, the crystal will have other circles of similar size as well, but the one to focus on is the one at the exact center of the crystal. Here marks the spot where the crystal was born.
Look at the center of this one (one of Mark's crystals):
The question is, what is that circle in the center?
The answer is: It is the original droplet upon which the crystal first formed.
Now that is the answer that I had heard a long time back. The originator, I think, was the incredible Wilson Bentley, the farmer-scientist of Jericho, Vermont during the early 1900s.
The problem was that it was never clear how the crystal could build upon the frozen droplet without erasing its boundary. We see this all the time in the lab - the droplet freezes, and then the vapor molecules add onto the crystal, turning it into a hexagonal prism, and then growing into whatever shape the conditions and crystal structure determine. There are absolutely no markings, borders, or interfaces that show what the original frozen droplet looked like. It is like pouring water into a cup that is half full: after you pour a little, you cannot tell that the cup once had less water. So, I never understood how Bentley's answer actually worked. Indeed, I think that until Akira Yamashita came along and pointed out how the boundaries of the original droplet could remain, nobody knew. His argument, which he proposed just a few years ago, involved the same curious process that we found in the corner pockets (see the previous post).
The process is sketched out in A-D below:
The frozen droplet under "a)" spreads an ice layer mainly along the top and bottom, making the cap and boot in 'b)". The molecules spreading across the flat surfaces migrate over the edges to the adjacent rim region, and plug into growth sites there. So the cap and boot spread out beyond the rest of the frozen droplet, as in "c)". The sides of the frozen droplet are essentially in a "vapor shadow" and receive almost no vapor: they hardly grow at all, and remain essentially frozen at their original size.
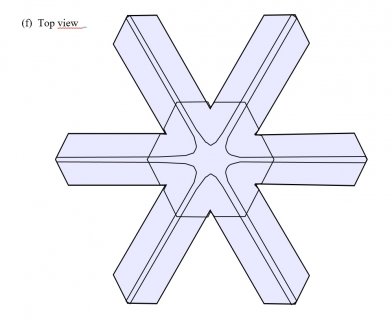
This process of the top and bottom (cap and boot) levels growing outward continues, making the crystal look like the empire fighter ships in Star Wars.
So, "c)" shows the two levels of every snow crystal. But the top and bottom levels are too close to each other. If one gets ahead of the other, it leaves the smaller one in a vapor shadow, just like the sides of the original frozen droplet. These other Japanese physicists found out how one side gets favored: It is all in the fall orientation. Remember that snow crystals are falling through the air, even as the air pushes them higher. Small, flat objects tend to hang in the air broad-wise, like a frisbee or flying saucer. The lower side (boot) gets a little more vapor, being upwind, and robs the top side (cap) of its fair share. So the lower level gets much bigger. Later, this frisbee flips back over, but it is too late for the stunted level: it remains forever stunted as the other level takes off, growing the six main branches, side-branches, and whatever features develop on its long journey to the ground.
But these stunted regions remain, including the sides of the original droplet. Not all snow crystals have this center. In many, the droplet gets erased as we observe in the lab. More on that in my next post. If you read this far, you now know something about snow that essentially nobody else does.
Finally, about those other circles on the crystal, the ones that look similar to the one in the center. These too are frozen droplets, droplets that had a startling run-in with the crystal and froze on contact. When they do this, we call them "rime".
-JN
(Crystal image is copyrighted to Mark Cassino, who let me repost here. The Bentley excerpt is from a poster I made years ago. Please do not reproduce without permission, thanks.)
Akira's Corner Pockets
May 10th, 2016The evanescent snow crystal
appears out of nowhere
The lines and boundaries
on its faces record a story
a story of a crystal's birth
a story of a crystal's life
But before the record vanishes
Who will hear its story?
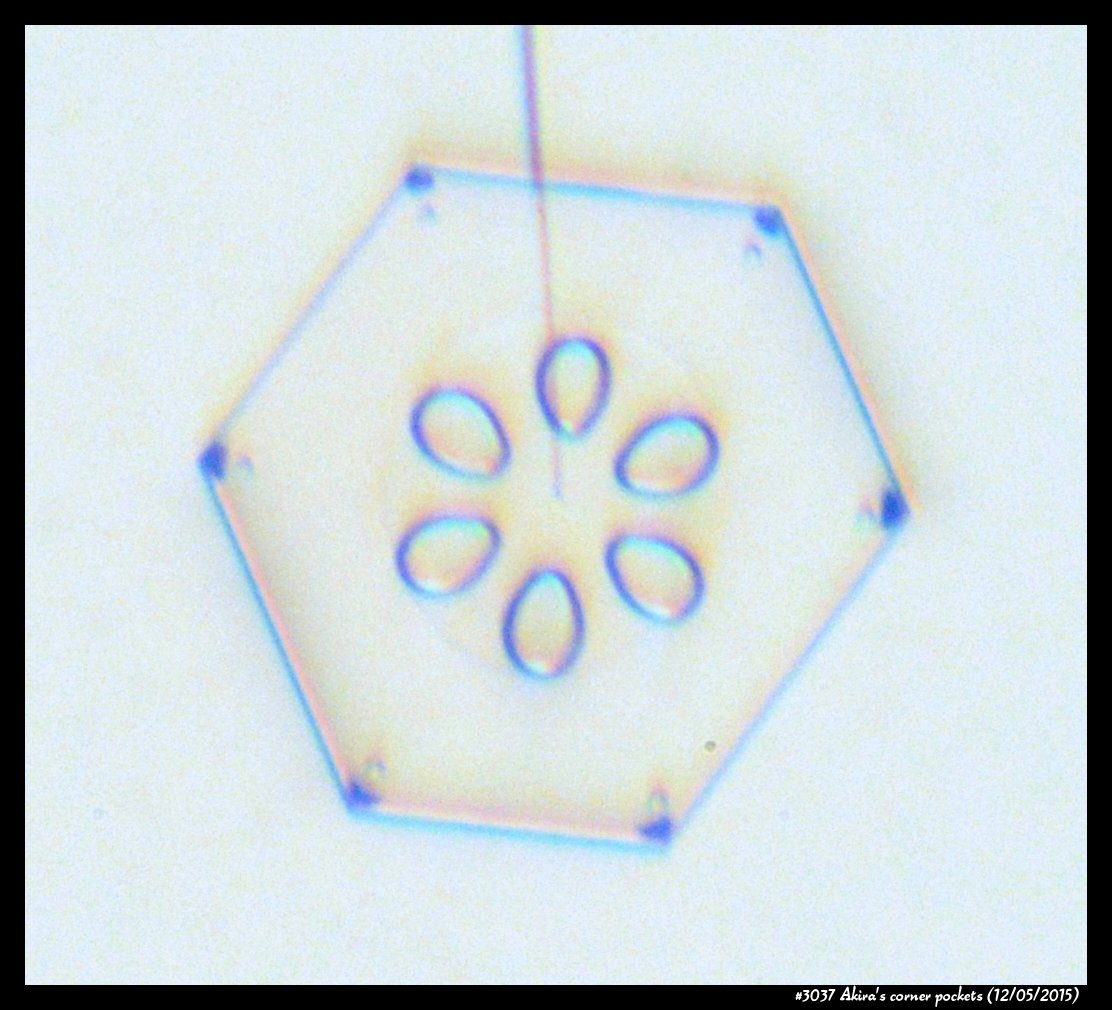
A few years back, a correspondent of mine, Professor Akira Yamashita of Japan, long retired, sends me an email. In the email, he had a document with words and pictures of some small crystals that he'd captured back in the 70s. They were small crystals, essentially freshly "hatched eggs" from the frozen droplets upon from they had started. But some had small pockets of air near their corners.
To those who have studied any sort of crystal growth and have some familiarity with crystal-growth theory, these corner air pockets, or "bubbles", were in impossible locations. They should not be there. Pockets will form near face centers, not corners. But Prof. Yamashita also had a theory about their formation. His theory first looked sketchy to me, but I appreciate hearing about new ideas, so over the following years kept revisiting his theory, getting to think that it had merit, and wondering if it had other applications.
Then, just this past year, in our own ice-crystal experiments, we did something that apparently had never been done to small ice crystals in the lab before. We slowly grew a crystal in air. And we cycled it from slightly growing, to slightly sublimating (i.e., shrinking in size), to slightly growing again. A cycle that must happen in some regions of cloud. And here is what we saw:
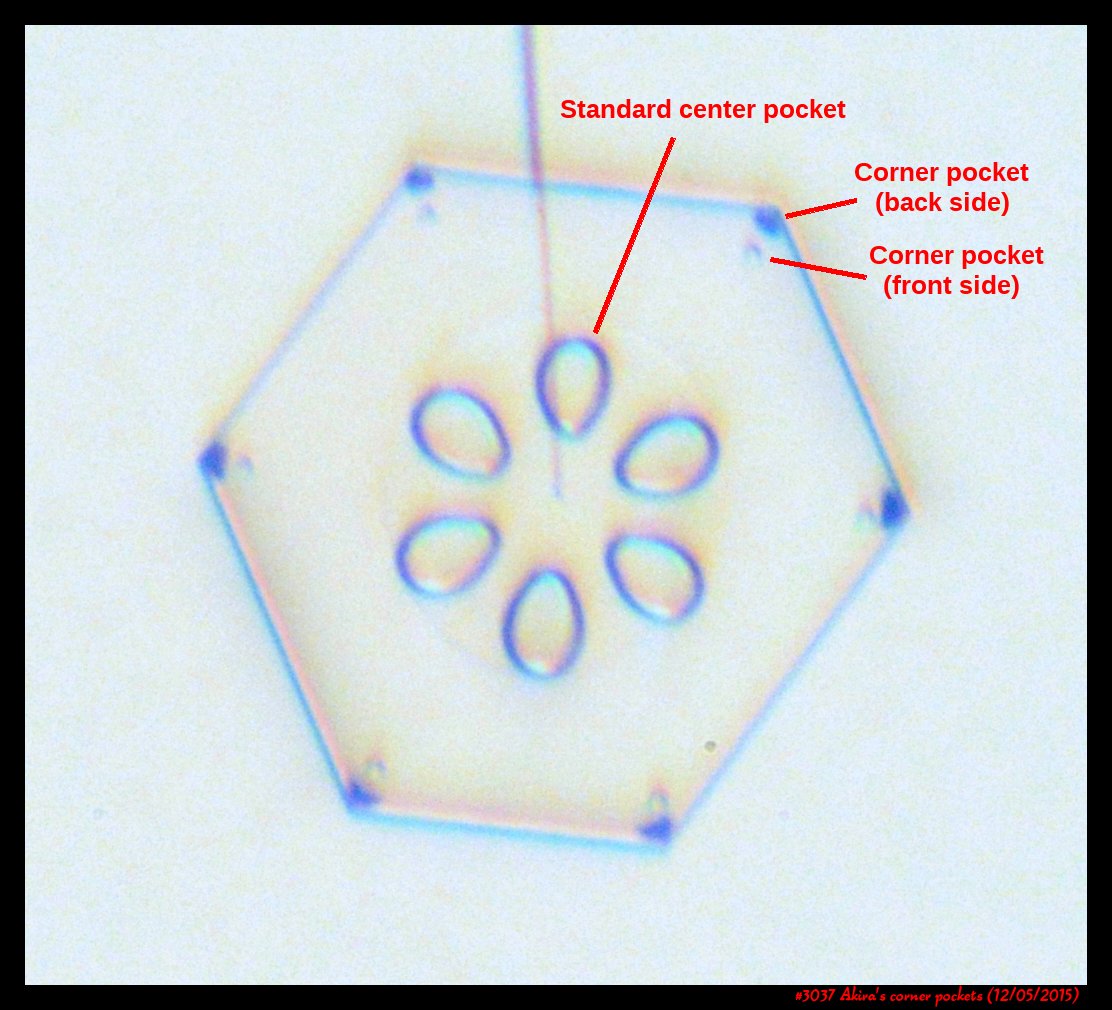
Corner pockets!
After the sublimating, the subsequent growth kept a permanent record of the sublimation cycle in the form of 12 corner pockets, one pocket for each of the 12 corners of the crystal. These are pockets of air, just like the six large 'petal-shaped' pockets of air you see nearer the center of the crystal. They are forever stuck in the crystal. Stuck there until the crystal, with all of its features, vanishes back to air.
After seeing this, we ran a few more experiments, and each time we slowly grew, then sublimated, then grew again, we got corner pockets. The name 'corner pockets' refers to their location when they are formed; namely at the corners, next to the crystal perimeter. However, they remain essentially fixed in position as the crystal grows, and this means that as the crystal perimeter expands outward, the corner pockets will appear further within the crystal. Analogously, the 'center pockets' shown above formed at the face centers, on the crystal perimeter, back when the crystal was much smaller.
As to the theory of their formation, and how the theory can explain other observable features of snow crystals, you'll have to wait for a subsequent post.
- Jon
One hundred twenty one forms of falling ice: the new snow classification system
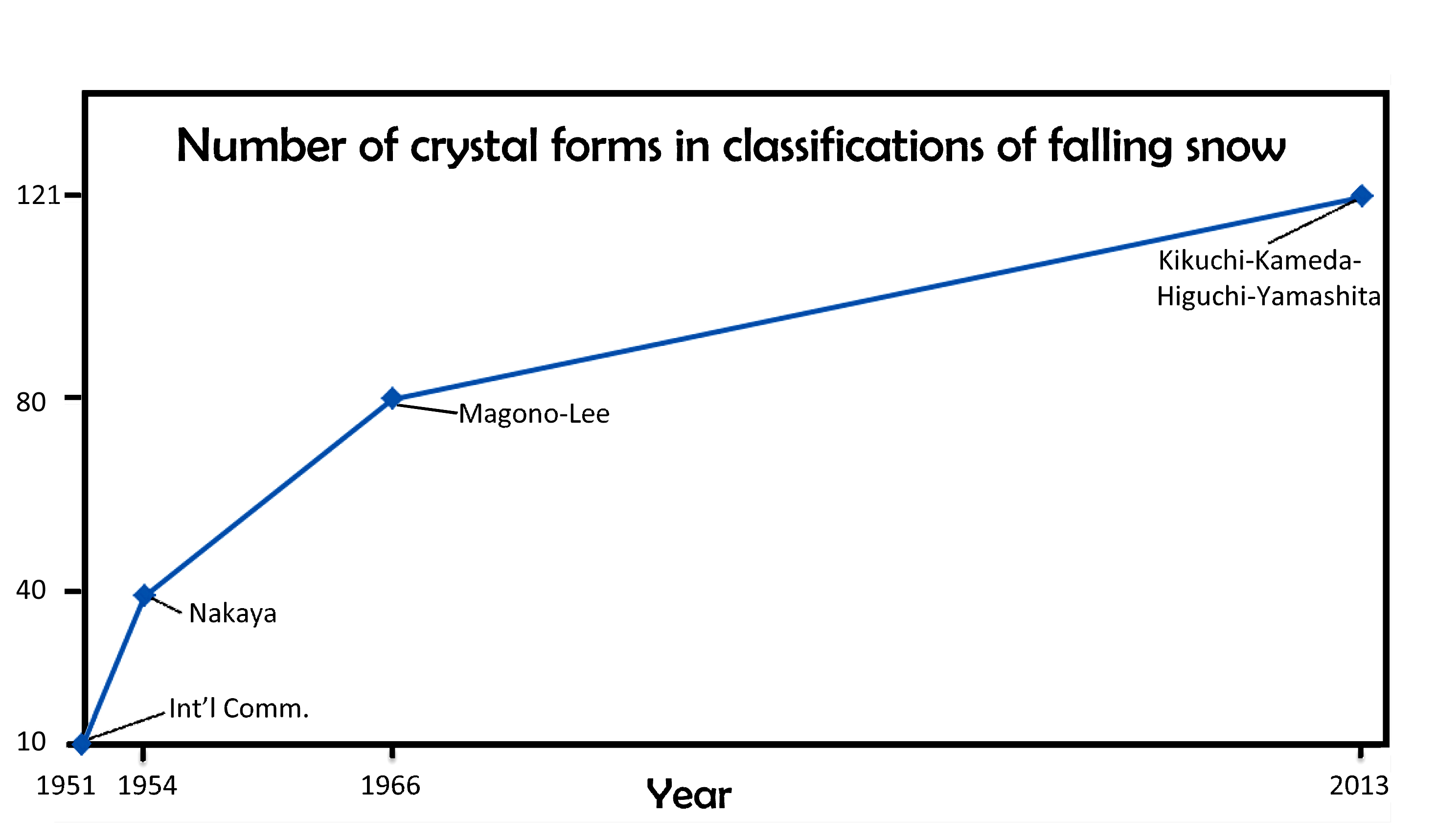
January 22nd, 2014When I last wrote about the classification of falling snow and ice (02/14/2010), I discussed the 1966 Magono-Lee system. At the time, this system was the most recent one, and as such, the one generally used in meteorology. And to those who wondered how many types of icy precipitation exist, Magono and Lee would tell you 80 types.
Although 80 sounds like a lot, Magono and Lee did not include at least one very common type as well as a few other interesting types. This major omission was snow-crystal aggregates, generally known as “snowflakes” by meteorologists. These are individual snow crystals that have stuck together during their fall and arrive as large open clusters of crystals, often with tens–to–hundreds of crystals in a big, round blob. (To meteorologists, a snow crystal is not a snowflake; rather, a snowflake consists of many snow crystals. It is like the difference between a trundled rock and a landslide.) In fact, the snowflake is probably the most common type of snow precipitation in most areas that receive snow. Several other omissions of the Magono-Lee that I mentioned in my 2010 posting included spearheads, seagulls, and the both the 18- and 24-branched forms.
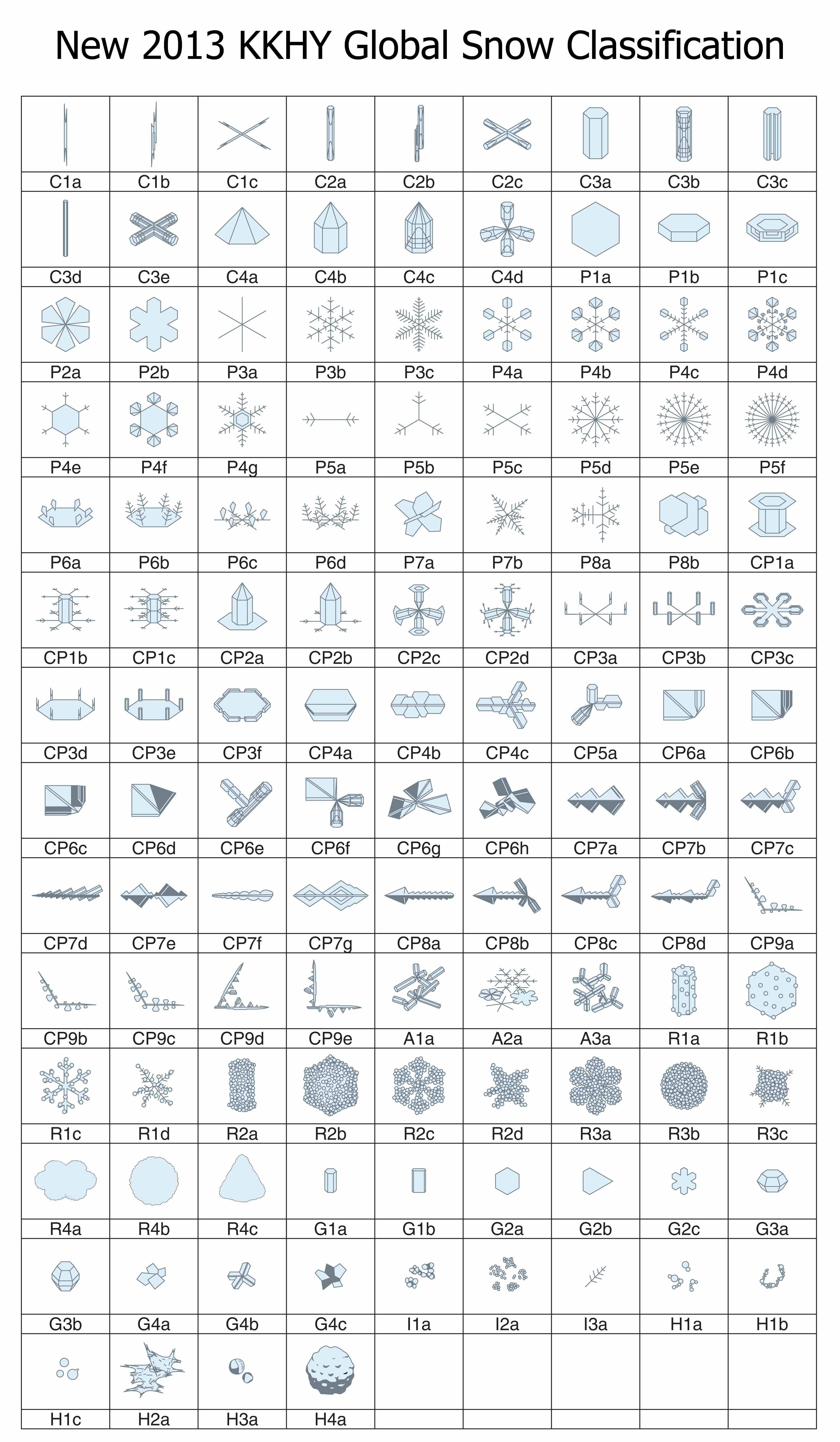
If you were waiting for someone to fix the classification, well wait no more: we now have a new classification scheme that includes all of these and more. The new system has not one, but three types of snow-crystal aggregate (snowflakes), which are given the symbol “A” (for aggregate). There is also four types of spearhead crystal (symbol “CP8”), five types of seagull crystal (symbol “CP9”), and both the 18- and 24-branched crystals (P5e and P5f). In all, the new system has added 41 new types. In one big graphic, here is the new system:
The architects of this new classification scheme published the above table in summer 2013*. There were four authors (K. Kikuchi, T. Kameda, K. Higuchi, and A. Yamashita), making it harder to name the system after the originators, but hopefully their names will become known, just as Magono and Lee’s have.
I will discuss various aspects of this new system in subsequent postings. But for now, I point out that the number of basic types has increased over the years, from 10, to 40, to 80, and now 121. When will it ever end?
Of course, it may just keep increasing. In fact, if we plot the numbers vs the years, and look at the pattern, we might conclude “never”.
Rime falling from branches
January 24th, 2013While riding my bike home the other day, I saw what appeared to be a patch of light snow.
It was the only such patch around. Looking closer, I could see that it consisted not of snow, but of chunks of partly melted rime deposits. (Note how the pieces are long and narrow, like little icicles.)
Just above were the branches of a huge Douglas Fir tree. Perhaps there had been an over-active squirrel or two up there. Other trees still had their rime. Hard to see why only this tree would have its rime fallen off.
- JN
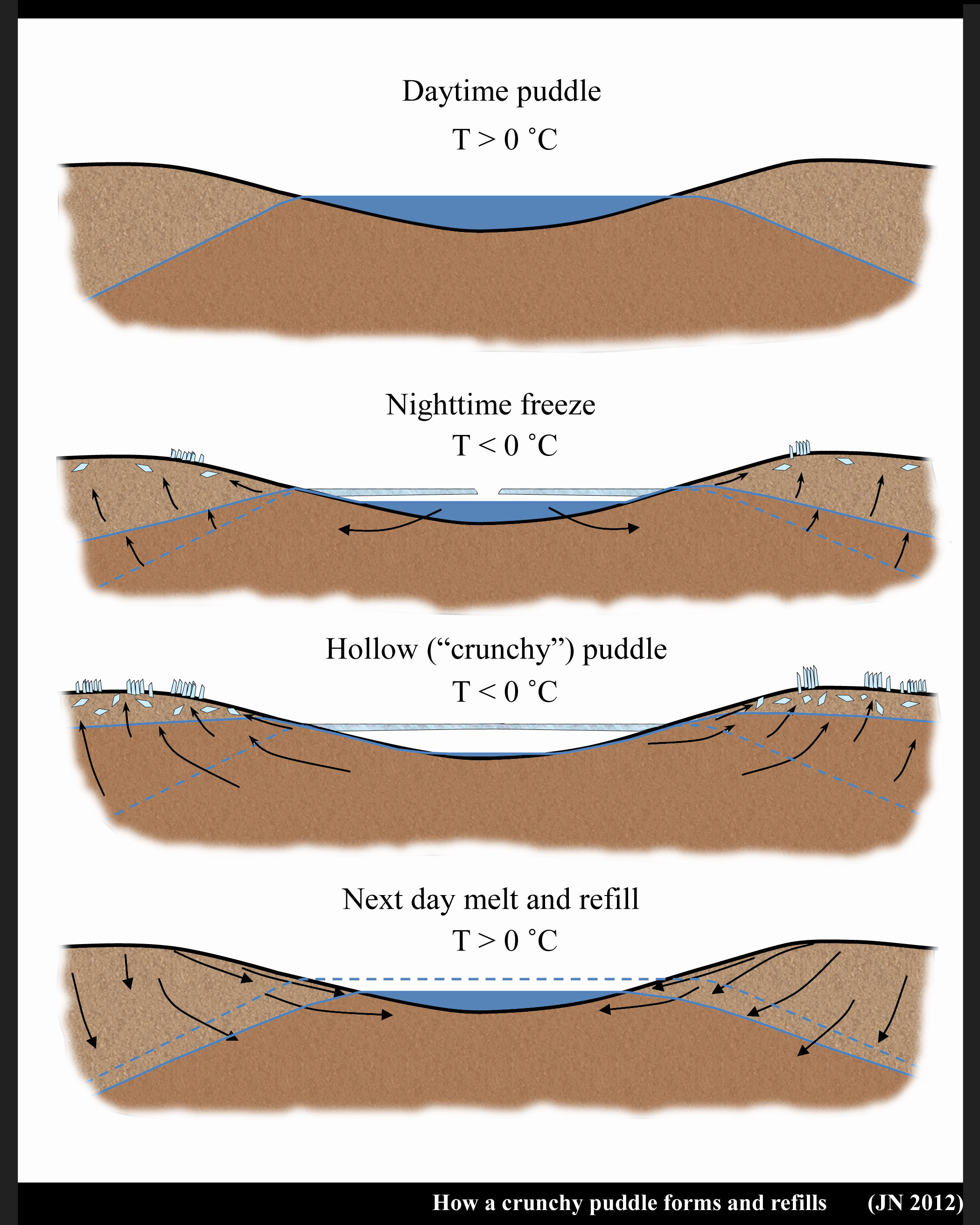
The Crunchy Puddle Puzzle
March 24th, 2012Who cares about crunchy puddles?
As a kid, I liked to stomp on hollowed-out frozen puddles to hear their crunch. Sure, I sometimes wondered, as many kids probably do, about what happened to all the water and why it happened only to some of the puddles. But such puzzles don’t linger in the mind of a kid for long.
Years later, while living in Japan, I rediscovered frozen puddles and saw their beauty in new ways. I saw new puzzles and revisited the old one about the missing water. A fellow ice-researcher I asked thought the water simply drained. But if they drained, how could I observe puddles in the same location morning after morning? How could the water come back without rain or snow? And why would they suddenly drain just as ice was forming? Something funny seemed to be happening. Searching the scientific literature, I found out that Kenichi Satake, also in Japan, had puzzled over the same thing. But he also offered a solution. His solution was published in Nature in 1977:
What he noticed was that a) the puddle water did reappear day after day, just as I thought, and b) their disappearance coincided with the formation of needle ice.
He pointed out that the freezing of the puddle coincides with the freezing of the top surface of the ground. When the ground freezes and ice needles form, water flows up to the base of the ice needles and push the needles up. That’s why they grow as columns. But the ground water must come from somewhere, and if a puddle is nearby, water will flow out of the puddle, through the ground, and deposit on the base of the ice needle. Even if the water must flow uphill.
I reproduce his sketch below.
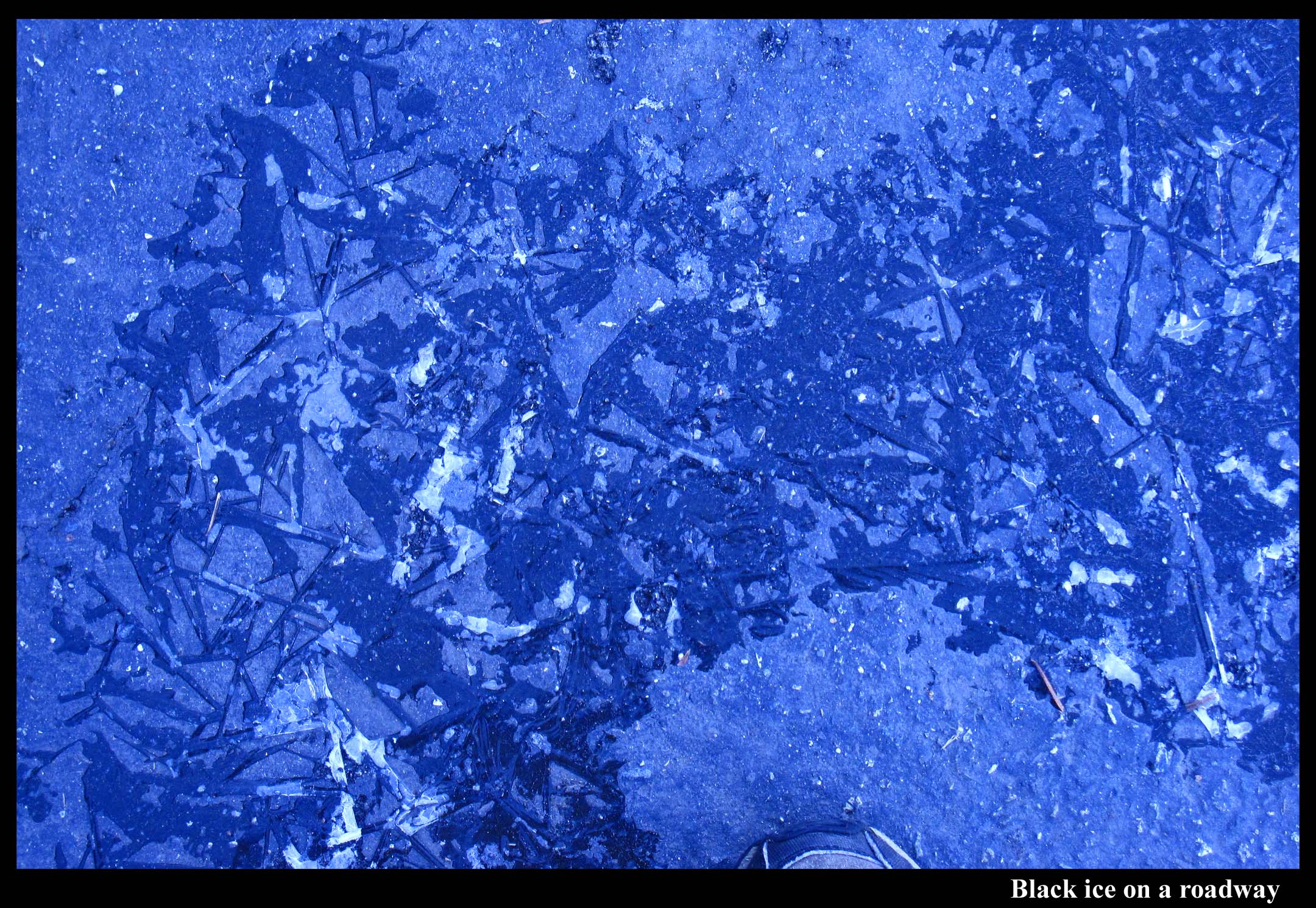
Black Ice
March 10th, 2012Black ice can form on sidewalks.
It can form in parking lots.
And it can form on small pieces of plastic.
To be “black”, the ice only has to be noticeably darker than its surroundings. And it can be darker if the ice attaches snugly to the underlying surface and is smooth on top. That is, the ice is black when it flows onto the surface like liquid water. The darkening effect is strongest when the underlying surface is rough because the water or ice fills the cavities that otherwise make the roughened surface a little whiter. As the image below shows, ice and liquid water darken a smooth surface as well, but the effect is weaker than that for the first three photos where the surface was rougher.
Black ice forms by water flowing onto the surface and then freezing. Black ice is black for the same reason that wet surfaces appear dark. In fact, black ice’s resemblance to liquid water is likely one reason it is so dangerous – though we’d hesitate about stepping onto smooth ice, we think little of stepping onto a slightly wet patch.
Finally got it!
February 8th, 2012For a few years now, myself and another ice-researcher have been trying to get a research grant from the National Science Foundation to study snow. Well, we just recently heard that the funding came through and I’ll be starting on the project in March. Yeah!
You might wonder what it is that we don’t already know about snow. Well, the better question is, what do we really know? The answer is “not much”. There’s been a lot of experiments in the lab, spanning nearly 100 years now, with many interesting results, but nothing really clear has emerged. Here’s one of the basic problems: In a typical cloud consisting of supercooled droplets and snow crystals, we can calculate the crystal’s rate of growth fairly accurately if we know the crystal shape. But it is extremely difficult to predict the crystal’s shape, and the rate of growth can change a lot with the shape. That’s the thing about snow crystals - they come in a bewildering variety of shapes, and we still have no clue as to why. And if the crystal isn’t surrounded by supercooled droplets, the situation is even worse.
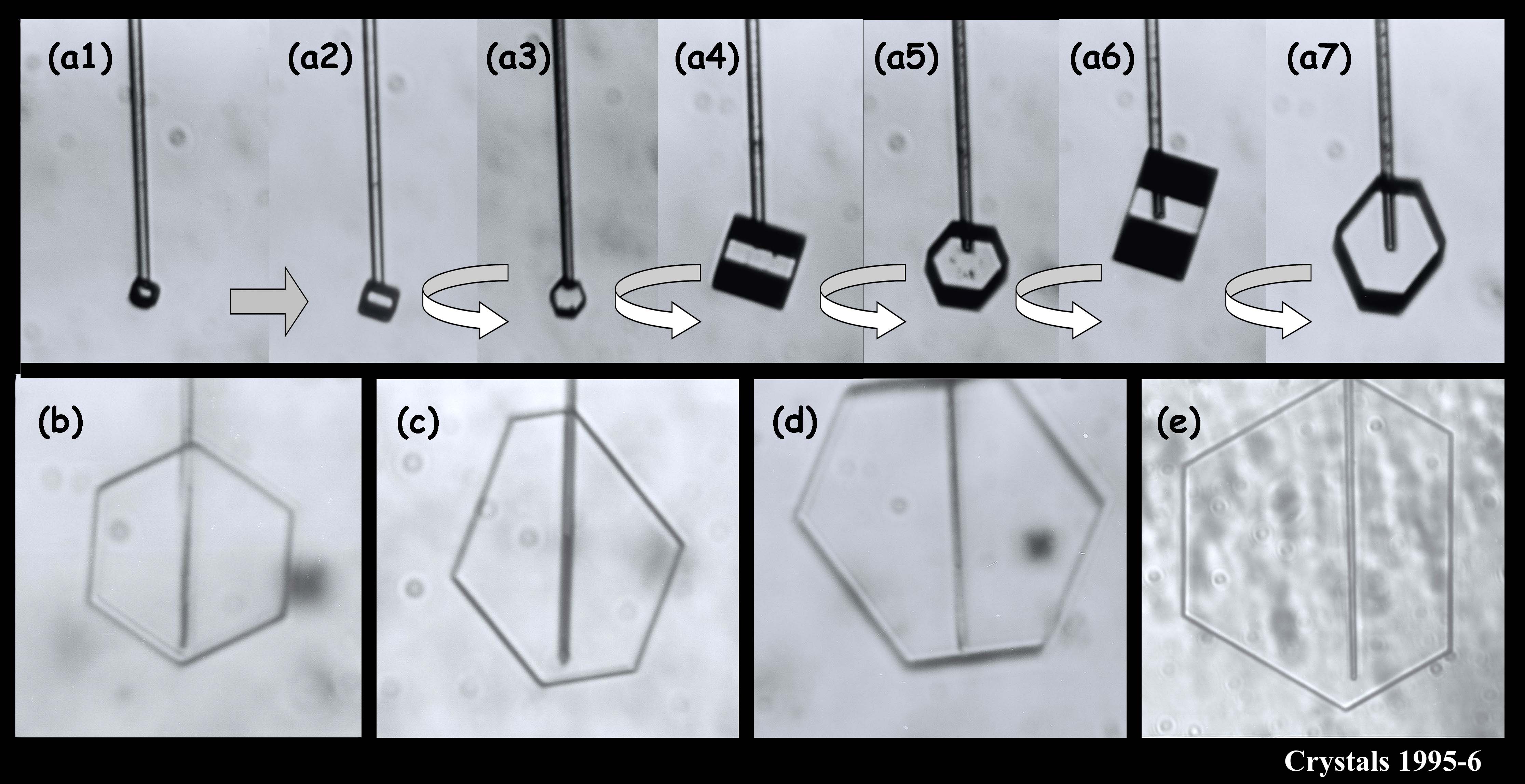
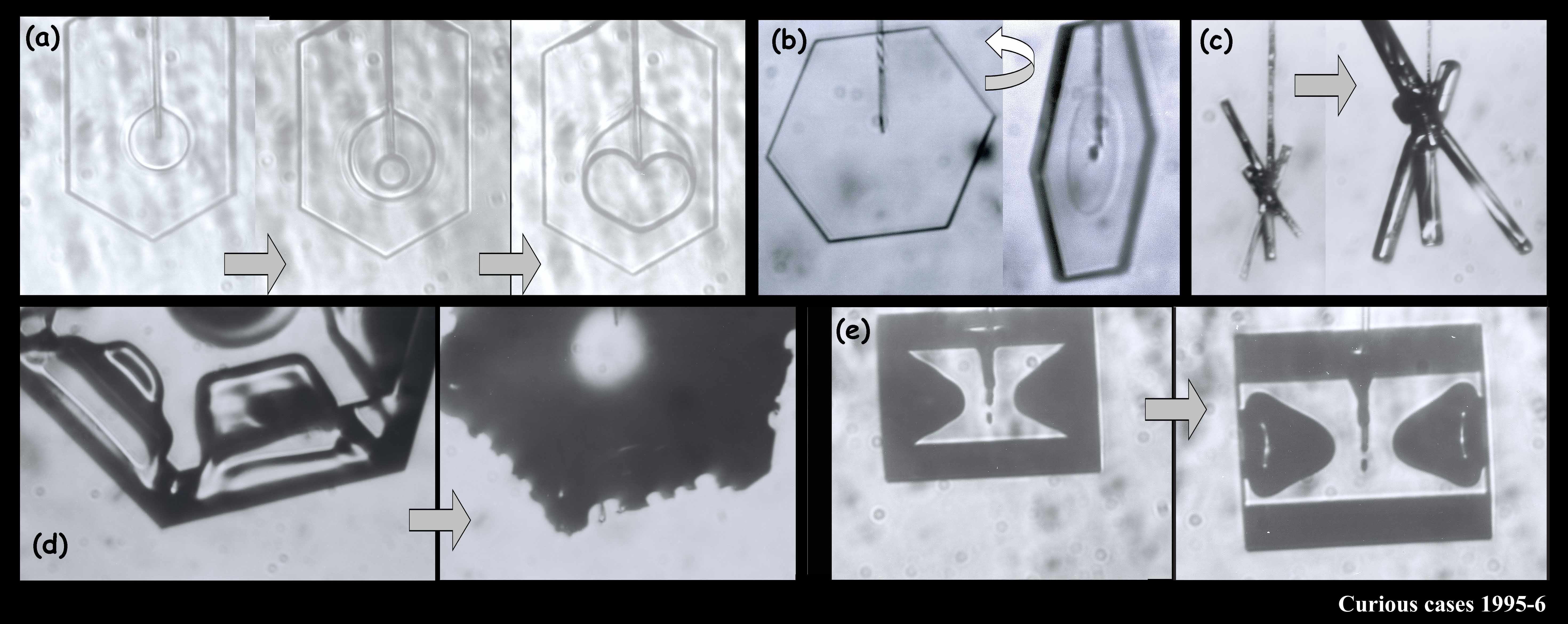
We argued in our proposal that the main reason we don’t have a clear picture about the development of snow crystal shape (and even growth rate) is because of problems with the experimental methods. Instrumental influences easily creep into the experiment and alter the crystal shape. We suggested a new method based on one I helped developed in 1994-1996. In that method, we grew single ice crystals from the tip of an ultrafine glass capillary (about 10x thinner than the typical hair on a person’s head). Some of the images below show what I mean about shape variety.
The crystals are all very small and compact because we were interested in growth with very low humidity. In the series (a1) - (a7), you see the crystal growing as I rotated the capillary to see all of the crystal faces. Sometimes I let a crystal grow for a week or more.
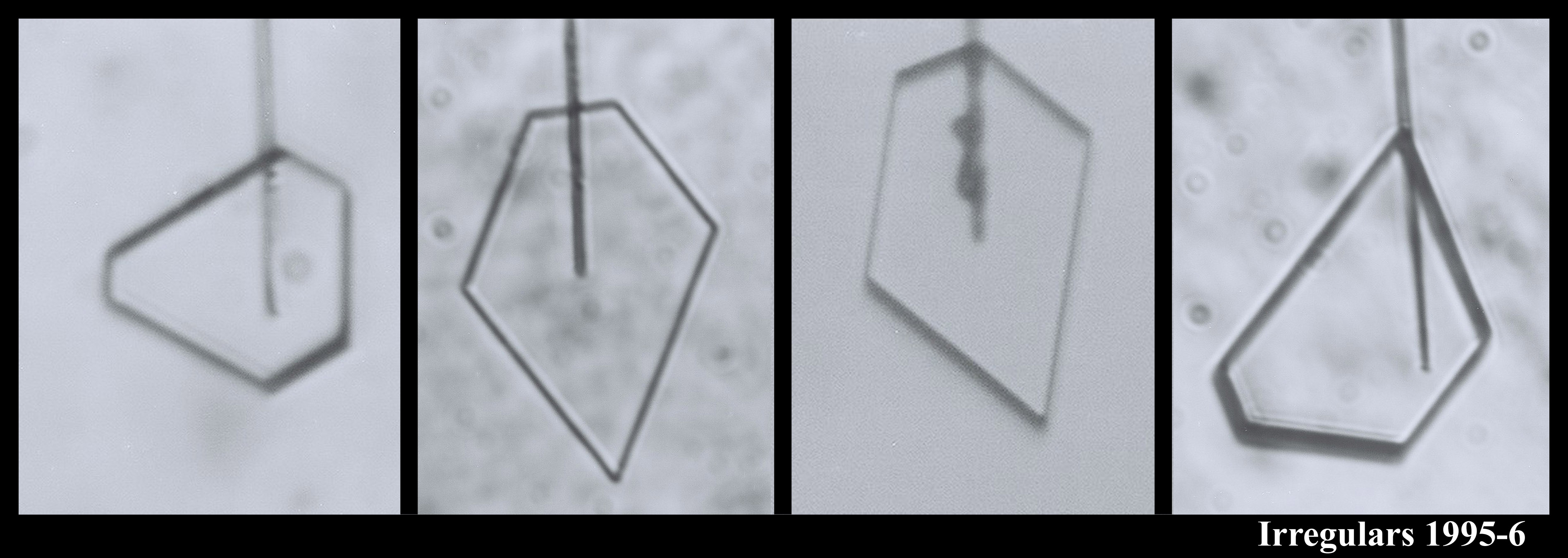
You can see that sometimes a crystal is 5-sided. But the angles between the prismatic faces are always multiples of 60 degrees. I don't think I'll ever see a 5-sided snow crystal that looks exactly like a pentagon.
The large crystal at the bottom left & middle was starting to melt. The melting formed cusps on the edges of the face. The one at the top right is called a bullet rosette. These crystals are very common in cirrus clouds and other very low-temperature clouds. The sequence at the upper left shows something we can do with a capillary: we can suck the crystal out from the inside. In that case, I put a vacuum on the other end of the capillary and a void started to form. Then, inexplicably, a crystal started growing inside the void! You never know what will happen in these experiments.
By this time next year, I hope to be able to show new crystals we grew. And sometime soon after that, have some reliable results.
-- Jon