Finally got it!
February 8th, 2012For a few years now, myself and another ice-researcher have been trying to get a research grant from the National Science Foundation to study snow. Well, we just recently heard that the funding came through and I’ll be starting on the project in March. Yeah!
You might wonder what it is that we don’t already know about snow. Well, the better question is, what do we really know? The answer is “not much”. There’s been a lot of experiments in the lab, spanning nearly 100 years now, with many interesting results, but nothing really clear has emerged. Here’s one of the basic problems: In a typical cloud consisting of supercooled droplets and snow crystals, we can calculate the crystal’s rate of growth fairly accurately if we know the crystal shape. But it is extremely difficult to predict the crystal’s shape, and the rate of growth can change a lot with the shape. That’s the thing about snow crystals - they come in a bewildering variety of shapes, and we still have no clue as to why. And if the crystal isn’t surrounded by supercooled droplets, the situation is even worse.
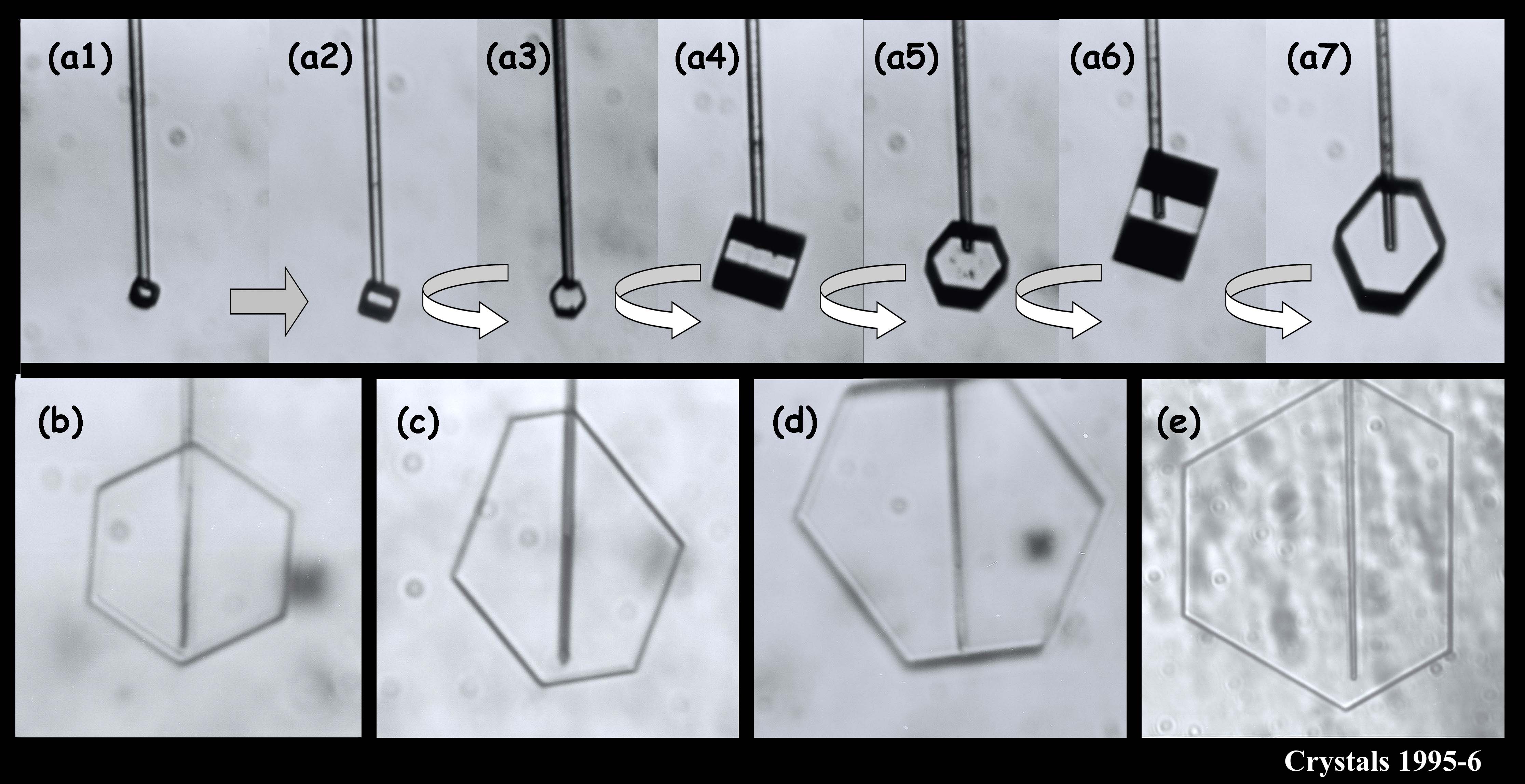
We argued in our proposal that the main reason we don’t have a clear picture about the development of snow crystal shape (and even growth rate) is because of problems with the experimental methods. Instrumental influences easily creep into the experiment and alter the crystal shape. We suggested a new method based on one I helped developed in 1994-1996. In that method, we grew single ice crystals from the tip of an ultrafine glass capillary (about 10x thinner than the typical hair on a person’s head). Some of the images below show what I mean about shape variety.
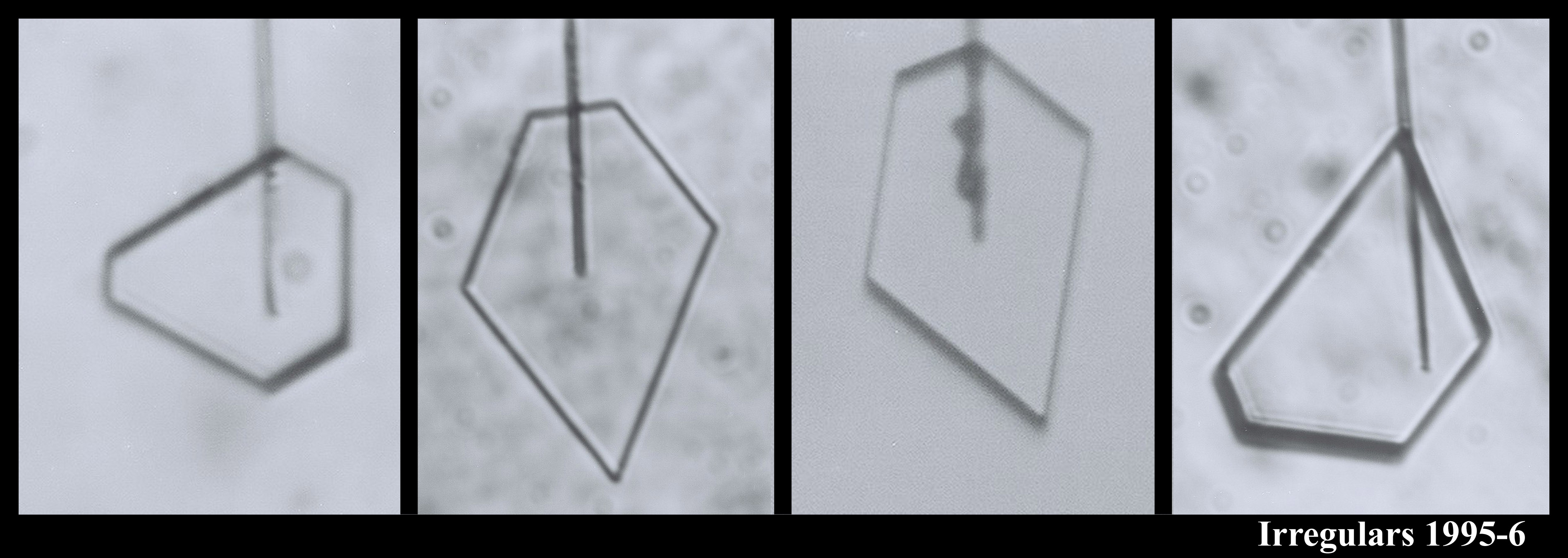
The crystals are all very small and compact because we were interested in growth with very low humidity. In the series (a1) - (a7), you see the crystal growing as I rotated the capillary to see all of the crystal faces. Sometimes I let a crystal grow for a week or more.
You can see that sometimes a crystal is 5-sided. But the angles between the prismatic faces are always multiples of 60 degrees. I don't think I'll ever see a 5-sided snow crystal that looks exactly like a pentagon.
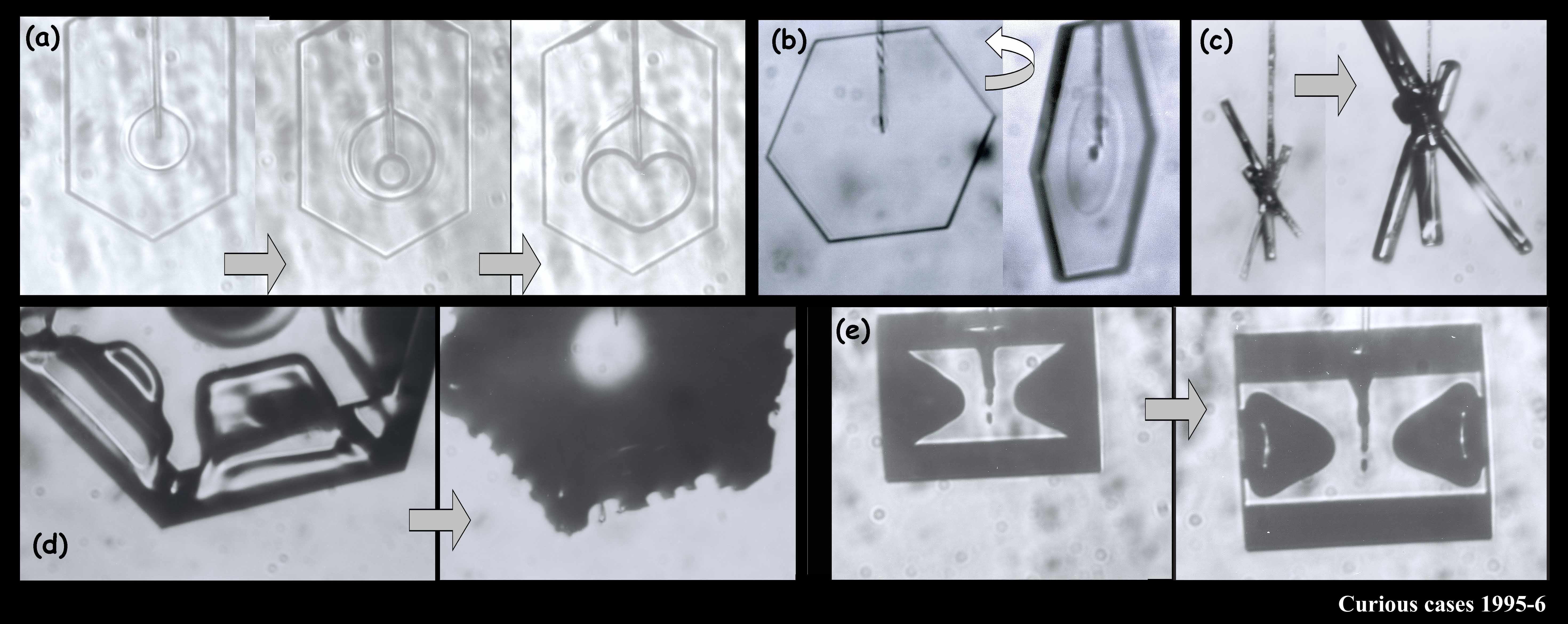
The large crystal at the bottom left & middle was starting to melt. The melting formed cusps on the edges of the face. The one at the top right is called a bullet rosette. These crystals are very common in cirrus clouds and other very low-temperature clouds. The sequence at the upper left shows something we can do with a capillary: we can suck the crystal out from the inside. In that case, I put a vacuum on the other end of the capillary and a void started to form. Then, inexplicably, a crystal started growing inside the void! You never know what will happen in these experiments.
By this time next year, I hope to be able to show new crystals we grew. And sometime soon after that, have some reliable results.
-- Jon
Caution!
February 1st, 2012Hoar. It's just a white coating on things, so why does it make everything look more interesting?
I saw this hoar coating on a plastic trash-can lid:
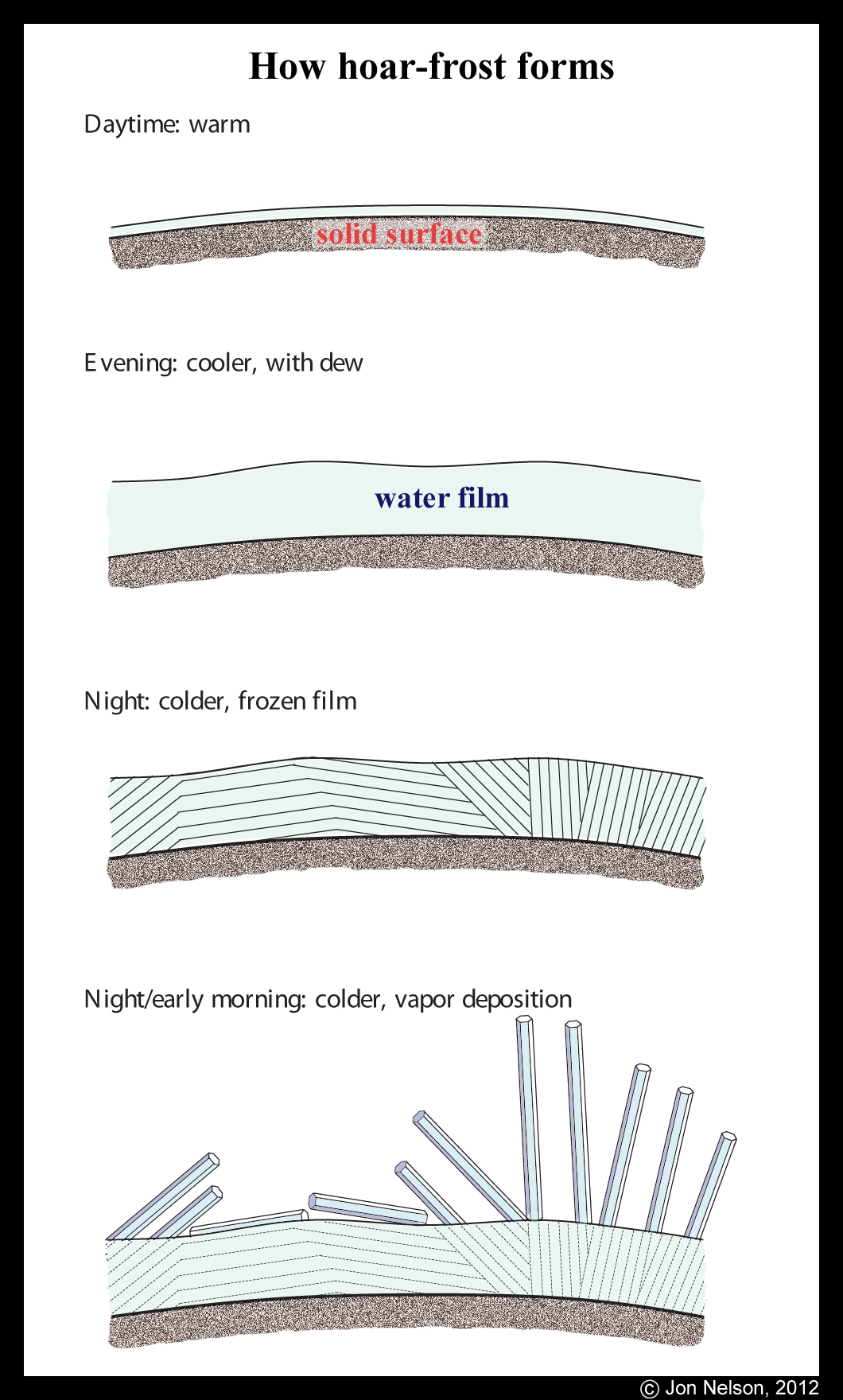
The hoar frost on the lid had various whirls, just like I've seen on the plastic surfaces of car door handles and side-view mirrors. This hoar was a little different though in that the crystals were definitely sticking up and not laying flat on the surface. Nevertheless, the fact that they show a pattern at all, and are not just randomly oriented, means that there must have been a liquid film of water that first froze to the surface. The film froze, producing a pattern of crystal orientations on the surface, and these orientations were not revealed until the hoar frost grew. Hurray for hoar!
Here's another warning:
The hoar crystals are longer on the raised lettering, particularly near edges. This is not because such places are further from the ground, but because they have more radiative cooling (due to their more expansive view of the sky) and can stick out into regions with a greater density of water vapor molecules.
If you click on the images, you can see the crystals a little better. But I forgot my tripod on this particular morning (I took the shots after I got to the office), and so the images aren't as crisp as my other close-up shots.
- Jon
Caution!
February 1st, 2012Hoar. It's just a white coating on things, so why does it make everything look more interesting?
I saw this hoar coating on a plastic trash-can lid:
The hoar frost on the lid had various whirls, just like I've seen on the plastic surfaces of car door handles and side-view mirrors. This hoar was a little different though in that the crystals were definitely sticking up and not laying flat on the surface. Nevertheless, the fact that they show a pattern at all, and are not just randomly oriented, means that there must have been a liquid film of water that first froze to the surface. The film froze, producing a pattern of crystal orientations on the surface, and these orientations were not revealed until the hoar frost grew. Hurray for hoar!
Here's another warning:
The hoar crystals are longer on the raised lettering, particularly near edges. This is not because such places are further from the ground, but because they have more radiative cooling (due to their more expansive view of the sky) and can stick out into regions with a greater density of water vapor molecules.
If you click on the images, you can see the crystals a little better. But I forgot my tripod on this particular morning (I took the shots after I got to the office), and so the images aren't as crisp as my other close-up shots.
- Jon
Slush Fingering and Other Pond Patterns
January 19th, 2012Here in the Pacific Northwest, we just had our first snowfalls of the season. On the weekend, we had 1 – 2 inches. This was followed on Wednesday by what the Seattle Times newspaper was calling a “megastorm”. But in the end, most areas in the area got only a few inches. Here in Redmond, we had 4 – 5 inches. And though the temperature barely dipped below freezing, I had several opportunities to observe snow patterns on the neighboring pond.
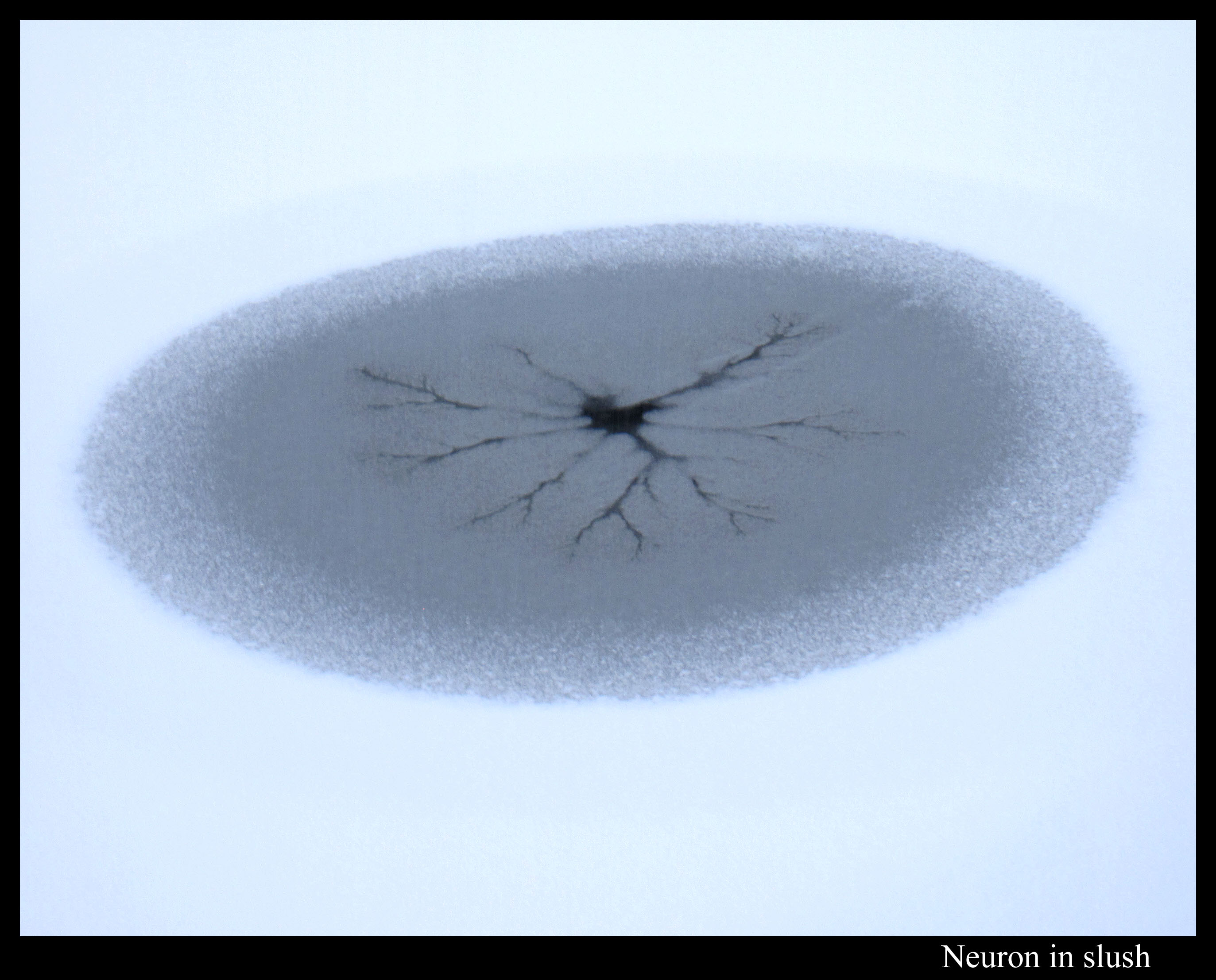
Or maybe I should say slush patterns. (Slush is a roughly uniform mixture of water and snow or ice.) Take the viscous fingering pattern I mentioned in my pond-ice post of a few days ago. Here is a similar type of pattern, except this looks like a neuron dendrite inside an egg.
Unlike the Boulder ice in that previous post, the ice layer on this pond was way too thin for me to walk on. Perhaps that’s a key to understanding why the patterns here instead had darker regions near their center. I don’t know. Anyway, the pond had a few such regions in which the water created dendrite-like fingering.
On the first snowfall, the pond had more circles with dark centers, but perhaps because the amount of snow was less, there was no discernable fingering near the centers, just dark centers caused by more uniform flooding.
In general, the basic layering was liquid below, clear and solid ice above, slush (or frozen slush) on top of the ice, and snow on top of the slush. (There can also be slush under a layer of solid ice.) The first snowstorm, though it had less snow, was preceded by colder weather. So, the clear ice was a little thicker and the slush layer thinner. Between the snowstorms, the ice melted and for awhile we had just slush on top of the liquid water.
The circular and fingering patterns arise when water gets pressed out of a small hole in the ice. The water then floods the ice as sketched below.
One Lone Crystal - 2012
January 16th, 2012This winter is turning out to be disappointing. We've had day after day of warm temperature, often barely dropping below freezing even in the evenings. What little snow we have gotten has been sloppy an d mixed with rain.
Last weekend we finally had a hard snow and a true blast of cold weather. While the detached garage in which I take these snow crystal photos had cooled down a bit, it still took till the next morning to finally drop below freezing inside the structure - even though it was well below freezing outside.
And so on Saturday morning I set up the camera and managed to get one, just one, snow crystal photo. The snow stopped just as I set up the rig and prepared to start photographing. Here's the one photo I did get - click for larger version.
And here we are - another warm day and rain on the way. Maybe more crystals will come soon...
Snow on a Freshly Frozen Pond
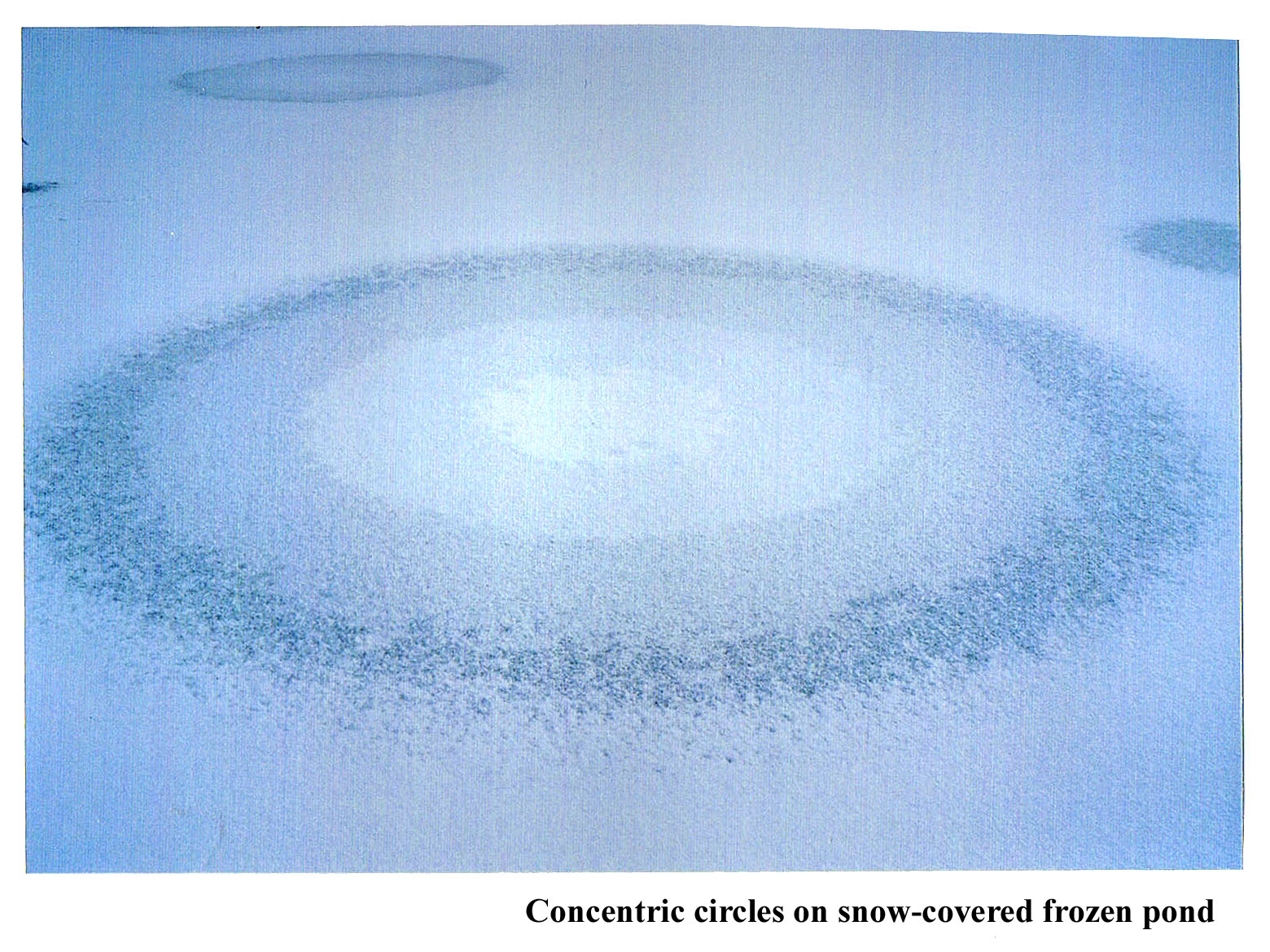
January 15th, 2012Back when I was doing post-doctoral work in Boulder, Colorado, Charlie Knight, the head of my lab, introduced me to strange ice phenomena. The most memorable one happened after the weather had been sub-zero for a few days and then we got some snow. When this happened, we stopped work and drove out to some shallow ponds to look at the patterns on the surface. Sometimes odd, concentric circles formed.
To see the size of the rings, check out the overview.
The guy in the background is Charlie. He is sawing through the ice to get a sample. Behind him are two visitors who came out with us that day. The ice was about 2" thick, if I remember right, and would make some cracking noises sometimes as we walked on it.
I don't know if he figured out the cause of the pattern. I never made any progress in understanding it. Anyway, what seems to happen is that water gets pushed out through a small hole in the ice, and the water apparently spreads out in a circular region. But why does the lightness of the ice change in nearly equally spaced, discrete steps? And why is it whitest in the center?
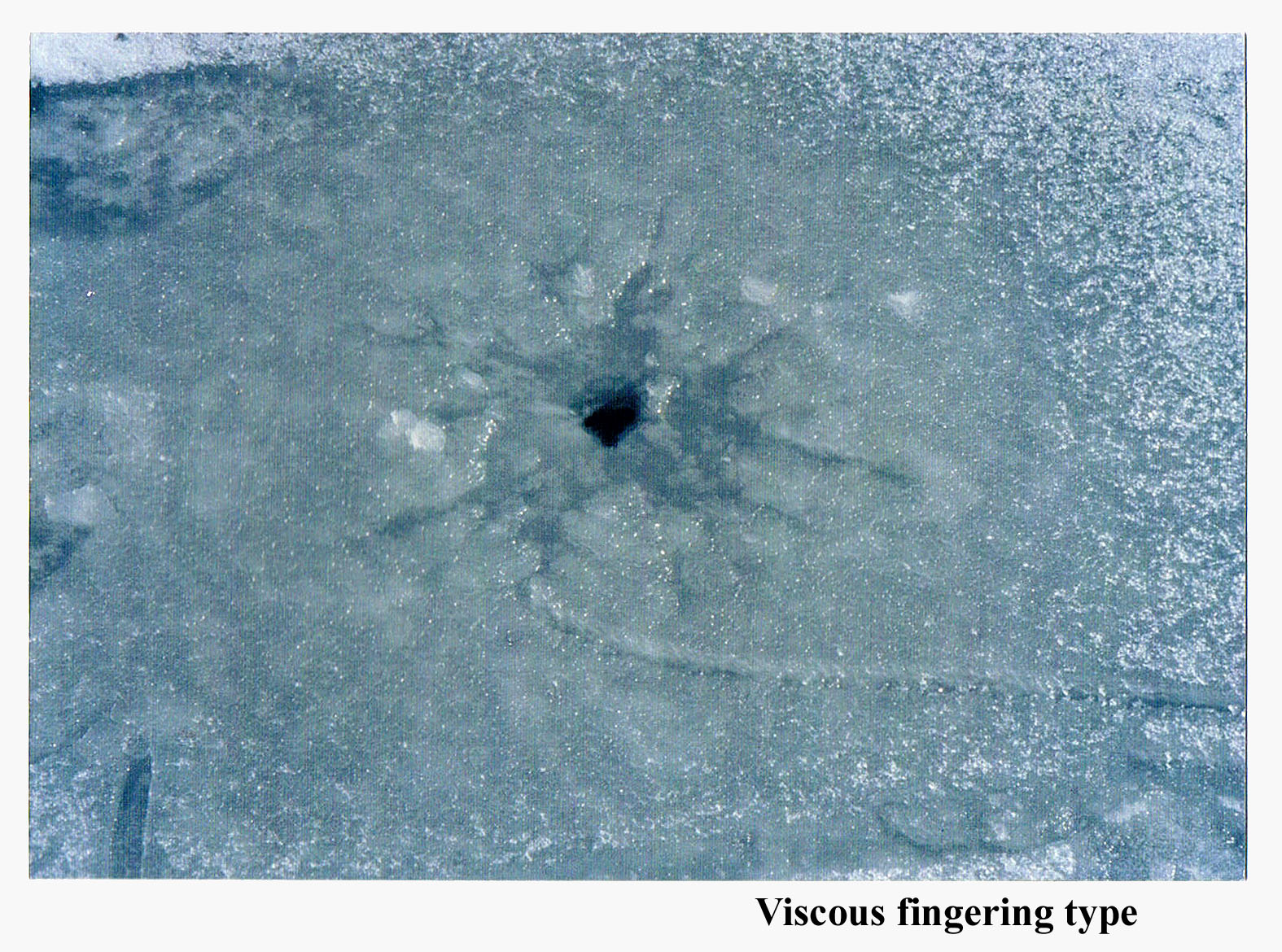
I figured that if water flooded over the ice in discrete steps (day-night temperature fluctuations, as we think happens with the pancake ice?), then the region in the center would be the darkest, not the whitest. For example look at this counterexample.
This shows the hole where water comes out, but the water floods outward in a ragged fashion, not like a concentric circle. This type of flow has been studied a lot in the laboratory and has the technical name "viscous fingering". Anyway, notice that the region near the center is darker, not whiter.
Any ideas about the concentric circles?
--Jon
Seeing Things in the Frost
January 15th, 2012In looking at the Cloud Appreciation Society's website recently, I started wondering; if they can have so much fun finding clouds that resemble various objects, why can't we do that for frost patterns?
Awhile back, I posted frost that looked like an eye ("Eyes and Dry Moats" Jan. 22, 2010). Here are a few other forms:
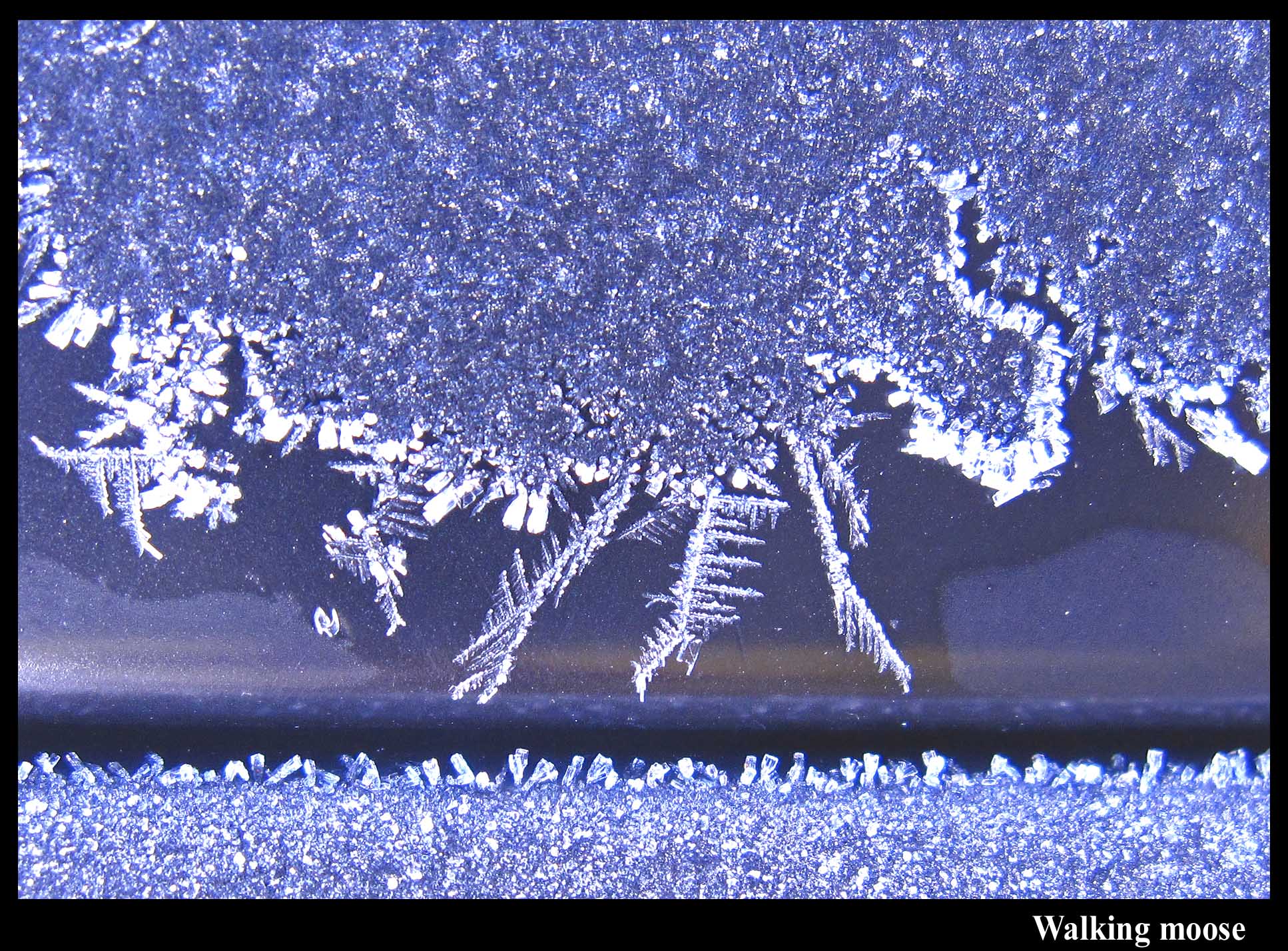
A moose.
A tree.
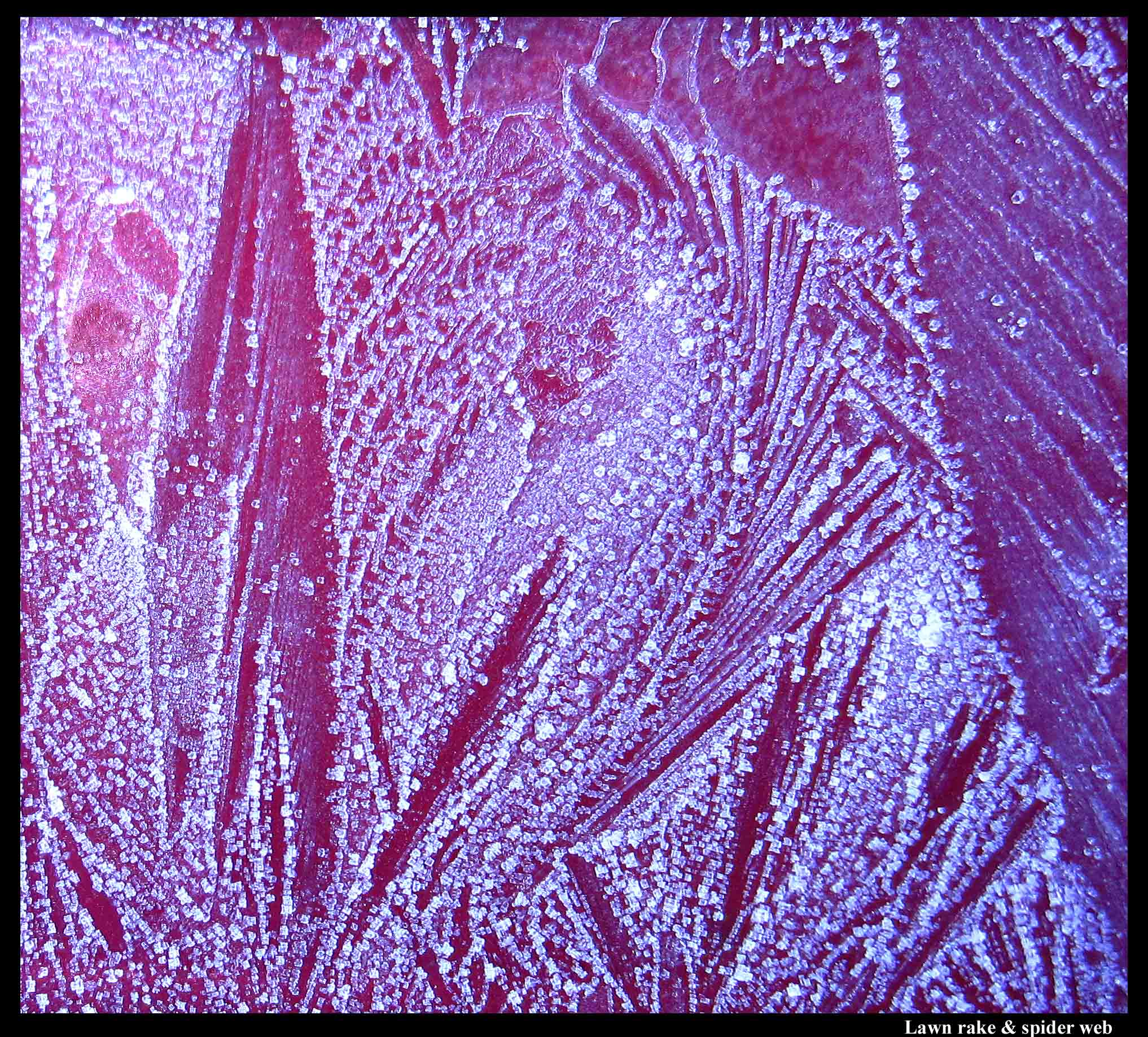
A lawn rake (or hand) and a spider web.
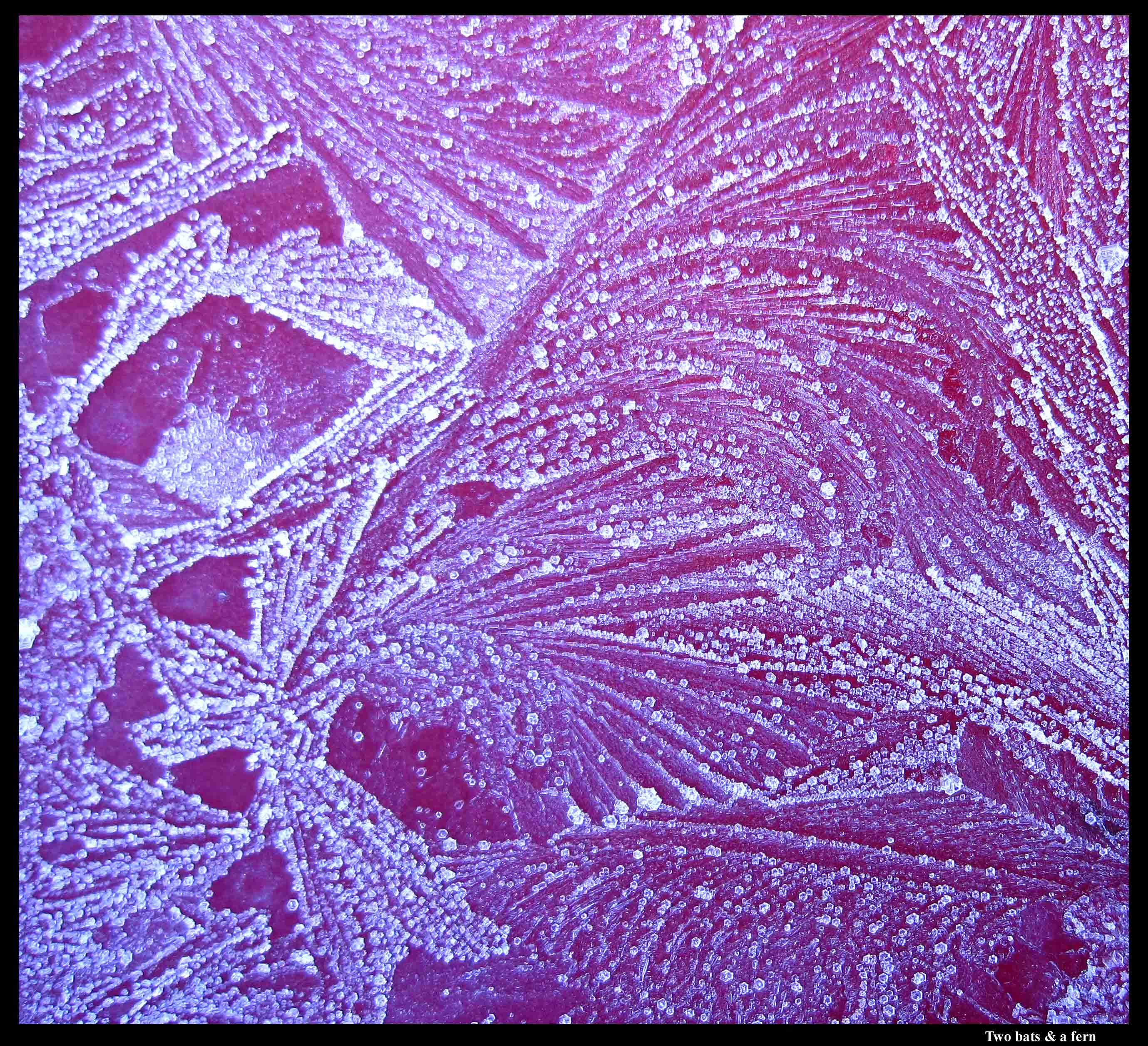
Two bats and some ferns?
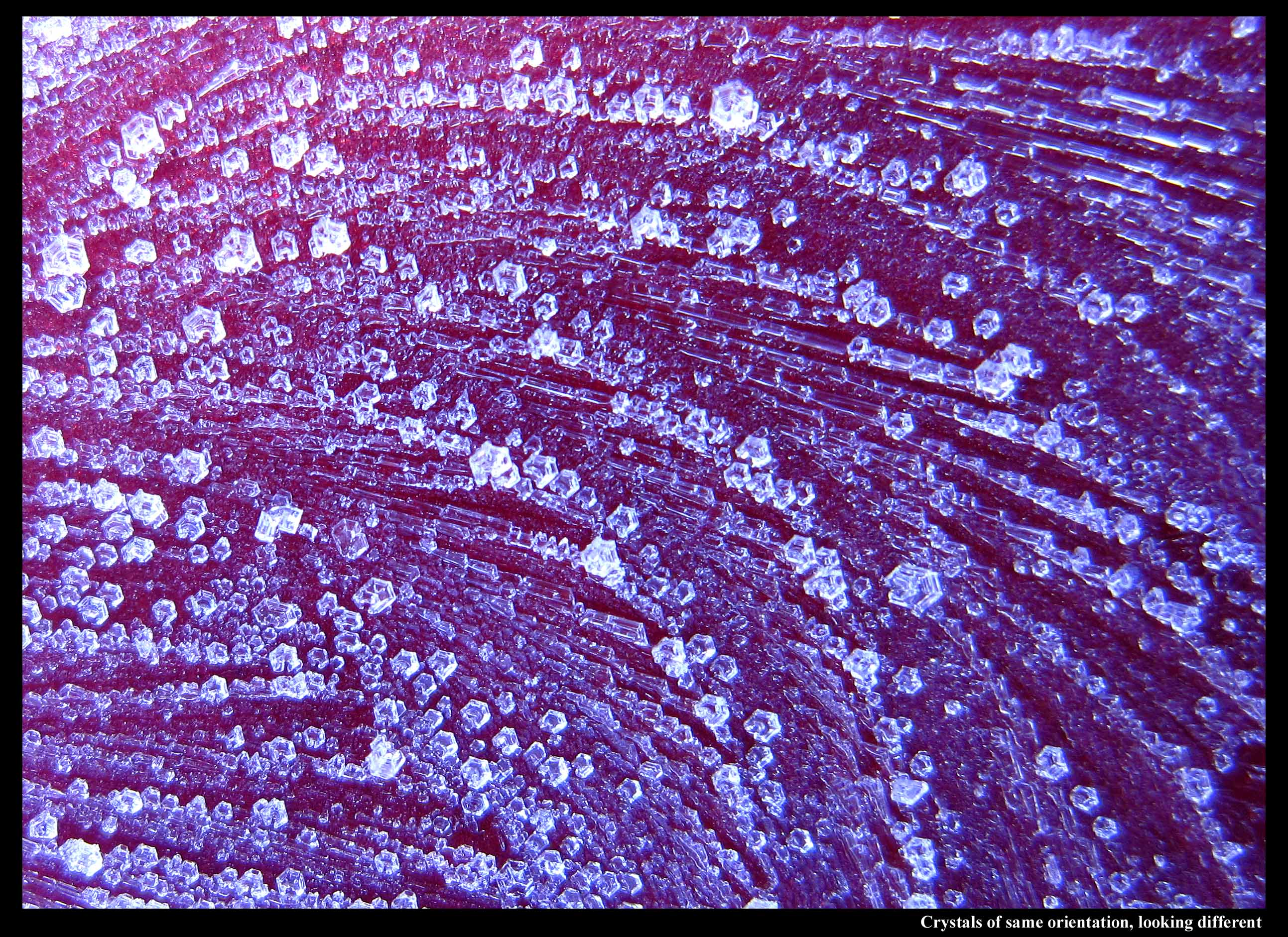
I noticed a few interesting things about the ferns in the above image. Look at the close-up below:
If you were to follow a line of crystals out one of the curves, and then take a ruler to the edges of the crystals, you will find that crystals at one end are slightly turned from crystals at the other. It's a slight effect, more easily seen in longer, curvy patterns, but it shows that when a finger of ice grew in the underlying water film, the crystal lattice actually twisted with strain as the finger curved. This was found out by experiments in the 1960s, so I was aware of this effect. But the other curious thing is that the line of crystals has two basic shapes: long but stubby ones and nearly hexagon-shaped crystals. They all have the same crystal orientation, but they look different. I hadn't noticed that effect before.
-- Jon
Fun with windshield ice
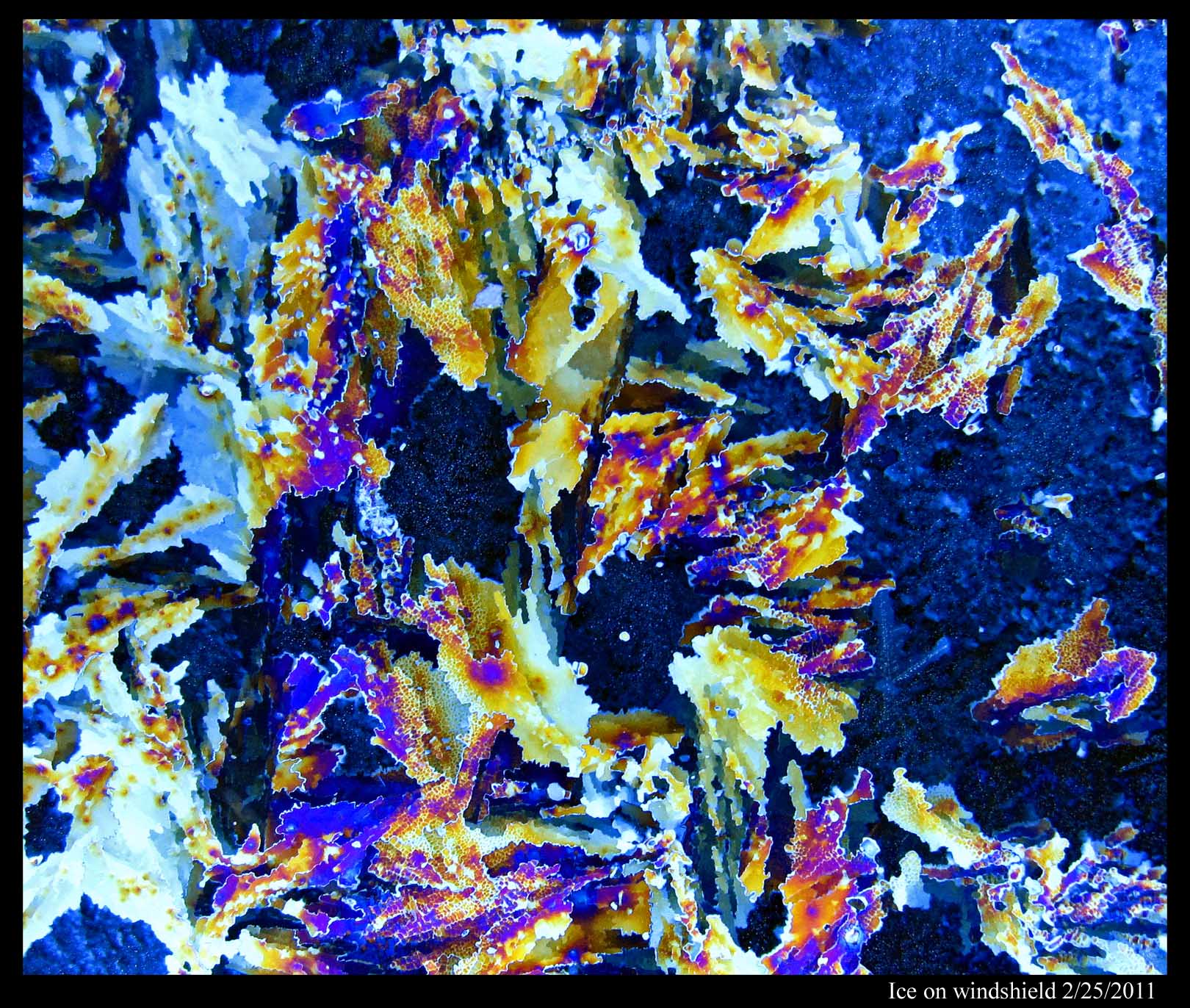
January 11th, 2012In my last post, I pointed out that you can determine the crystal orientation in a film of ice by looking at hoar-frost that sprouts from its surface. Here's another way, but it only works if the ice is on glass. For example, here's ice on my windshield, as seen through crossed polaroids:
To get colors, the ice must be sufficiently thick. You can play around by spraying a mist of water onto your windshield (or some other sufficiently cold pane of glass), letting it freeze, looking through the polaroid sheets, and then spraying some more if you don't see colors you like. One polaroid sheet must be in front of the ice, the other behind the ice. And you need to cross the sheets. You can tell when the sheets are crossed because a bare pane of glass will be black between crossed polaroids.
Two things determine the color: the thickness of the ice and the crystal orientation. So, the boundary between different colors marks the boundary between different crystals. Black regions usually mark regions where the crystal is "basal" orientation; that is, you are viewing the ice crystal lattice from the same angle that you are viewing the nice dendrite crystals that Mark posts here. And where the color changes gradually, you are seeing where the ice thickness is changing gradually.
Of course, don't try this while driving!
-- Jon
Choppy waves
January 11th, 2012I thought this hoar frost pattern looked like rough seas. I see choppy, cusp-like waves down there.
Not having any pictures of rough seas, I hopped in our bathtub and kicked my legs around to make waves. Perhaps you can see a little resemblance.
However, the processes that caused the cusp-like, choppy wave pattern in the frost are completely different from those in the bathtub. Look more closely at the hoar-frost pattern:
It just goes to show you that hoar frost is never as simple as you’d think. Though from a distance it might look like white whiskers, up close it shows unexpected patterns. These patterns reveal something about how the crystals strained, twisted, and competed with each other.
Though it is true that the hoar crystals we see are built of deposited vapor molecules (invisible water molecules once floating in the air that happened to strike and freeze onto a cold surface), the story of hoar has an earlier beginning. In the beginning, the surface already had a thin film of liquid water. See the top panel in the sketch below.
Book Signing at the Kalamazoo Nature Center
November 30th, 2011On Saturday, December 10, the Kalamazoo Nature Center will host the 2011 Buy Local Art & Gift Fair. Lots of artists this year and a really fun event. I will be on hand to sign copies of The Story of Snow from 1-3 PM. For more details, click here.
If you are in the area, stop by!